Fig. 4. A model for the effects of TF and normal diets on TGF-β receptor partitioning between lipid raft/caveolae and non-lipid raft microdomains.
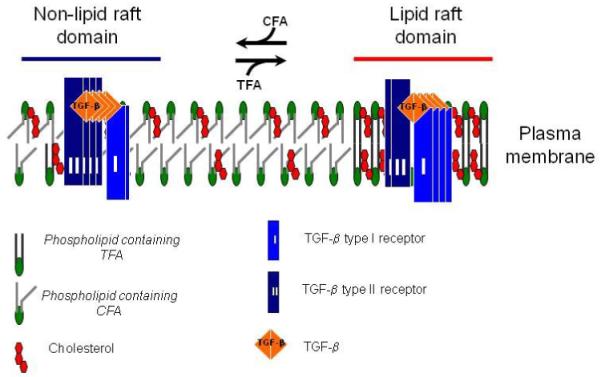
In cells (including vascular cells), two major TβR-I -TβR-II complexes (Complex I and Complex II) present on the cell surface (23,24,29,30). Complex I and Complex II are mainly localized to the non-lipid raft and lipid raft/caveolae microdomains of the plasma membrane, respectively. The numbers of TβR-I and TβR-II molecules (blue and black rectangles, respectively) in Complex I and Complex II shown in the model are arbitrary and intended to indicate that Complex I and Complex II contain TβR-II >TβR-I and TβR-I >TβR-II, respectively. The ratio of TGF-β binding to TβR-II compared to TGF-β binding to TβR-I (TβR-II/TβR-I binding) can be determined by 125I-TGF-β affinity labeling (23,24,29,30). Trans-fatty acid (TFA, derived from the TF diet) incorporation into phospholipids increases formation of, or stabilization of, lipid rafts/caveolae, facilitating accumulation of TβR-I and TβR-II in the microdomain where TGF-β binding to the receptors is incapable of inducing Smad2-dependent signaling but does cause receptor internalization and degradation, and thus attenuated cellular responses. Cis-fatty acid (CFA, derived from the normal diet) incorporation into phospholipids results in increasing formation of, or stabilizing, non-lipid raft microdomains where TGF-β binding to the receptors induces Smad2 signaling which leads to cellular responses. Both lipid raft/caveolae and non-lipid raft microdomains, which contain TFA moieties, in mice fed a TF diet are relatively unstable compared to those in mice fed the normal diet. This may explain the profoundly decreased levels of TβR-I and TβR-II in mice fed a TF diet as compared with those in mice fed the normal diet.
