Abstract
Gastrointestinal involvement occurs in about 70% to 90% of histoplasmosis cases but is usually not the initial manifestation. We present the case of a 52-yearold HIV-positive woman who presented with gastrointestinal symptoms and an apple-core lesion on CT scan of the abdomen. The patient had been diagnosed with histoplasma colitis eight months earlier and was started on long-term itraconazole therapy. However, she prematurely discontinued treatment. A colonoscopy during the present hospitalization revealed a 3.5-cm mass, biopsies of which revealed Histoplasma capsulatum. In the present report, we discuss the differential diagnosis of apple-core lesions in the colon and the importance of keeping histoplasmosis on the differential diagnosis, especially in endemic areas like the Ohio River valley. It is equally important to ensure compliance with treatment of histoplasmosis, as well as close follow-up, as progression to colonic obstruction while on medical management has been reported.
Keywords: AIDS, Colonoscopy, Fungal infections, Intestinal obstruction, Malignancy
INTRODUCTION
Gastrointestinal involvement occurs in 70% to 90% of the cases of histoplasmosis[1] ; however, it being the presenting manifestation is rare. Colonic histoplasmosis on presentation in immunocompromised patients can present a diagnostic challenge to clinicians, especially given that the other differentials are opportunistic infections and malignancy. The present report illustrates the importance of maintaining a high level of suspicion for gastrointestinal histoplasmosis in immunocompromised patients, particularly in endemic areas.
CASE REPORT
A 52-year-old HIV-positive woman presented to the hospital with complaints of diarrhea, abdominal cramps, nausea and fever of 3 weeks’ duration and vomiting of 2 days’ duration. She complained of three to four liquid to semisolid bowel movements per day that were without mucous and were associated with abdominal cramps. She had noticed black, tarry stools the day prior to admission; and at previous clinic visits, she had complained of small amounts of bright red blood per rectum with defecation. Her fevers were intermittent, with recorded temperatures between 101°F and 105°F, and were associated with night sweats, chills and headaches. The day before admission, she had two episodes of vomiting that contained undigested food but no blood. Her past medical history was pertinent for a diagnosis of disseminated histoplasmosis and histoplasma colitis in March 2009, which was treated with suppressive itraconazole therapy (200mg/d, twice daily). For her HIV, which was diagnosed in 2006, she was on lamivudine/ abacavir and lopinavir/ ritonavir, as well as prophylactic trimethoprim-sulfamethoxazole and azithromycin.
On admission, her heart rate was 103/min; temperature, 101.3°F; respirations, 18/min; and blood pressure, 134/84mm Hg. Pertinent findings on physical examination included mild suprapubic to mid-abdominal tenderness without any palpable mass lesion, guarding or rigidity of the abdomen. Her meningeal signs were negative, and cranial nerve function was intact. Her CD4 count and HIV viral load two weeks before admission were 50 /mm3 (9%) and 804,000 copies/mL, respectively. Laboratory values included hemoglobin, 11.6 g/dL; peripheral white blood cell count, 3,470/mm3 with a normal differential; and platelet count of 186,000/mm3. Liver enzymes and kidney function tests provided normal results. Urine showed greater than >600mg/dL of protein, trace bacteria but was negative for leukocyte esterase and nitrite. Subsequent urinalysis, however, did not show persistent proteinuria. A chest radiograph and computed tomography (CT) of the head without contrast were normal. In addition, abdominal radiographs revealed a normal bowel gas pattern.
Blood, cerebrospinal fluid, sputum and stool were obtained for cultures. Urine and serum histoplasma antigens were sent for analysis. An abdominal CT scan with contrast [Figures 1a and 1b] revealed a 4-cm apple-core lesion involving the ascending colon with areas of thickening in the cecum and the transverse colon associated with regional lymphadenopathy. There was abnormal stranding and adenopathy in the mesentery surrounding the mesenteric vessels. These CT scan findings were highly suspicious for malignancy; thus a tissue diagnosis was pursued. At this stage, the differential diagnoses included colonic malignancy, inflammatory bowel disease, lymphoma, mycobacterium avium complex (MAC), tuberculosis and histoplasmosis.
Figure 1a.
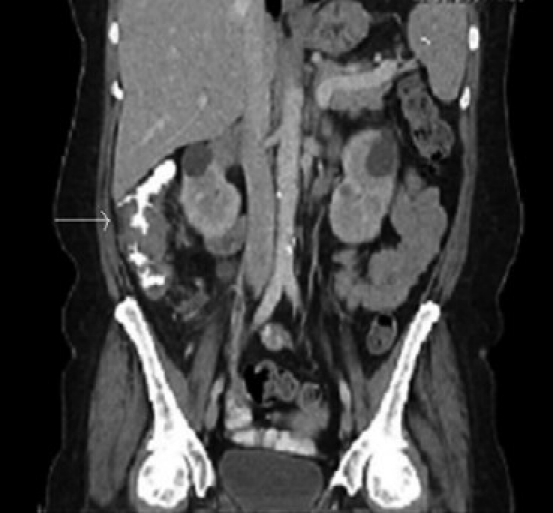
CT scan of abdomen with contrast with apple-core lesion in ascending colon (coronal view)
Figure 1b.
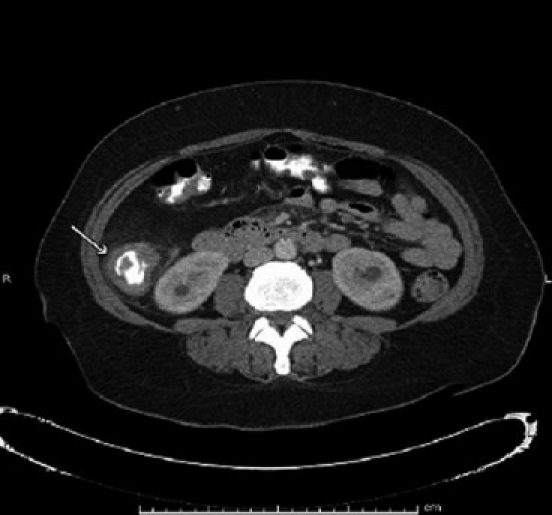
CT scan of abdomen with contrast with apple-core lesion in ascending colon (horizontal view)
Colonoscopy revealed a nonbleeding 3.5-cm mass [Figure 2] with erythematous and friable mucosa in the ascending colon, corresponding to the apple-core lesion seen on the CT scan. Partial obstruction with a single nonbleeding 8-mm ulcer in the distal descending colon with mucosal edema was also identified. The overall endoscopic impression did not favor malignancy. The biopsy was positive for small (2-5 μm) intracellular yeast forms with occasional narrow budding characteristic of Histoplasma capsulatum [Figures 3a and 3b]. Blood, cerebrospinal fluid (CSF) and stool cultures were negative. The patient was started on intravenous liposomal amphotericin B, and nasojejunal feedings to maintain nutrition. The urine histoplasma antigen ultimately revealed a positive result, at 25.2 units. A bone marrow biopsy was contemplated in view of the anemia and leucopenia on admission. However, in view of the positive biopsy and strongly positive urine histoplasma antigen, it was reasoned that a bone marrow biopsy would not alter management. Hence the patient was treated with liposomal amphotericin B for a total of two weeks, with a good response to therapy in the form of improving symptoms and hematological parameters (hemoglobin, 12.8 gm/dL; WBC, 4,210/mm3; and platelet count, 225,000/mm3 at discharge). Subsequently, intravenous itraconazole 400mg every 12 hours for 5 days followed by long-term suppressive therapy with 200mg of oral itraconazole twice daily was administered. The patient's HIV therapy was continued throughout the course of her hospitalization. Close follow-ups with the clinics of infectious diseases and gastroenterology were arranged.
Figure 2.
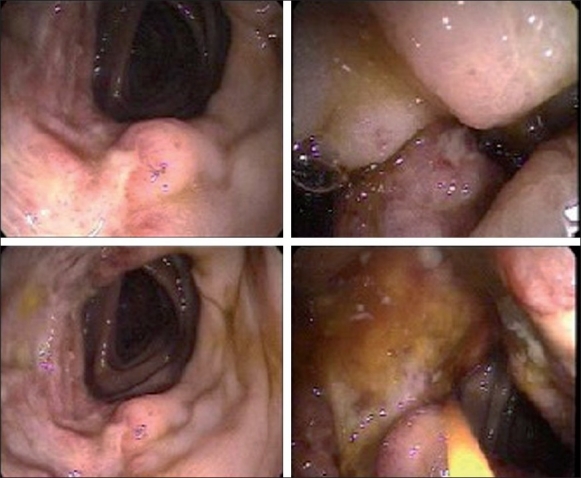
Colonoscopic images of multiple ulcerative lesions in the ascending colon causing incomplete obstruction
Figure 3a.
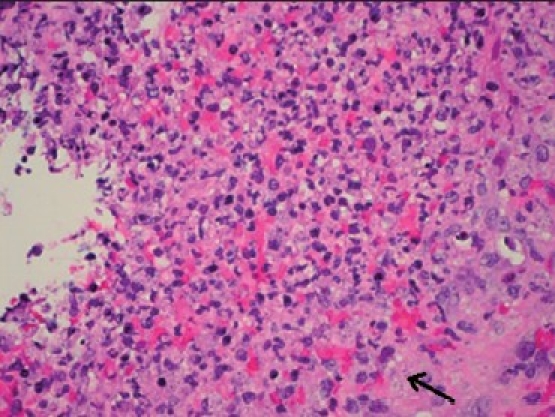
H and E stain of ascending colon biopsy demonstrating intracellular, 2–4-μm, oval, narrow-based, budding Histoplasma capsulatum cells
Figure 3b.
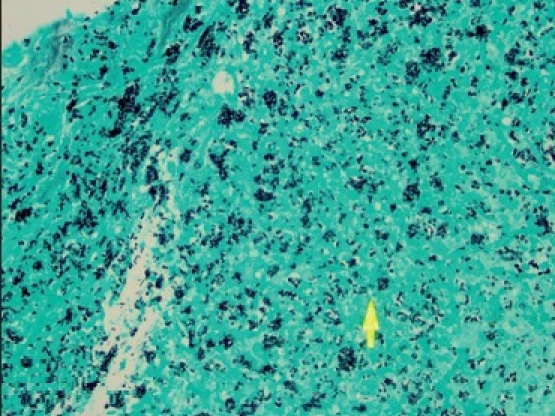
Gomori's methanamine silver stain of ascending colon biopsy demonstrating intracellular, 2–4-μm micro meter, oval, narrow-based, budding Histoplasma capsulatumcells
DISCUSSION
Histoplasma capsulatum, originally described in 1905 by Samuel Darling, MD, is endemic to the Ohio, Missouri and Mississippi river valleys.[1] Histoplasma is a saprophytic dimorphic fungus growing as a mold in nature but as yeast in host tissue or culture media. Gastrointestinal (GI) involvement occurs in 70% to 90% of the cases of disseminated disease[1] ; however, symptoms are usually not evident on initial presentation. GI infection by histoplasma is considered to be a manifestation of hematogenous spread of the fungus.[1,2] Four distinct pathological forms have now been recognized: 1) subclinical with microscopic clusters of macrophages in the lamina propria; 2) plaques and pseudopolyps caused by fungus-containing macrophages; 3) tissue necrosis and ulceration that leads to abdominal pain, diarrhea and bleeding; and 4) localized thickening with inflammation of the bowel that can mimic malignancy or Crohn's disease.[1]
Immunocompromised patients,[2] including those with HIV and common-variable immunodeficiency, can have a broad initial differential diagnosis when presenting with gastrointestinal histoplasmosis. In the present case, the patient had a prior diagnosis of histoplasma colitis, diagnosed on endoscopy eight months before this presentation. She had been started on itraconazole therapy but was not adherent. On this admission to the hospital, her symptoms with the associated new apple-core lesion on CT scan were particularly of concern for the possibility of colon cancer. However, after endoscopic biopsy, this was ruled out.
This case illustrates the variability of gastrointestinal symptoms and findings that can occur with opportunistic infections in immunocompromised patients. Gastrointestinal histoplasmosis symptoms can range from tongue or palatal ulcers, diarrhea and bleeding to intestinal obstruction and perforation, depending on the histological layer of the bowel that is involved.[3] Ileocecal involvement is commonly observed in histoplasmosis, possibly secondary to the excessive lymphoid tissue in this region of the gastrointestinal tract. Cases of colonic histoplasmosis presenting with mass lesions suspicious for malignancy have been documented in previous reports, with the diagnosis of histoplasmosis usually made on pathological examination of the operative specimen.[4–7] A previous report highlighted the fact that intestinal histoplasmosis can progress to complicated lesions, including strictures, despite adequate medical management.[8] The recommended treatment for histoplasmosis is liposomal for two weeks followed by long-term oral itraconazole treatment (200mg, twice daily). It is also pertinent to note that endoscopic persistence of histoplasma organisms on histology despite negative cultures has been noted in the literature, which supports the need for long-term itraconazole therapy. Our patient did not realize the importance of the treatment course for her histoplasma colitis and prematurely discontinued oral itraconazole, which led to disease progression.
CONCLUSIONS
Histoplasmosis should be included in the differential diagnosis in immunocompromised patients with gastrointestinal symptoms, especially in endemic areas, and appropriate education and follow-up arranged to prevent disease progression. It is equally important to educate patients about complications of intestinal obstruction despite being on adequate treatment.[8]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Lamps LW, Molina CP, West AB, Haggitt RC, Scott MA. The pathologic spectrum of gastrointestinal and hepatic histoplasmosis. Am J Clin Pathol. 2000;113:64–72. doi: 10.1309/X0Y2-P3GY-TWE8-DM02. [DOI] [PubMed] [Google Scholar]
- 2.Kane S, Brasitus T. Histoplasmosis capsulatum as a cause of lower gastrointestinal bleeding in common variable immunodeficiency. Dig Dis Sci. 2000;45:2133–5. doi: 10.1023/a:1026680317424. [DOI] [PubMed] [Google Scholar]
- 3.Assi M, McKinsey DS, Driks MR, O’Connor MC, Bonacini M, Graham B, et al. Gastrointestinal histoplasmosis in the acquired immunodeficiency syndrome: Report of 18 cases and literature review. Diagn Microbiol Infect Dis. 2006;55:195–201. doi: 10.1016/j.diagmicrobio.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Cimponeriu D, LoPresti P, Lavelanet M, Roistacher K, Remigio P, Marfatia S, et al. Gastrointestinal histoplasmosis in HIV infection: Two cases of colonic pseudocancer and review of the literature. Am J Gastroenterol. 1994;89:129–31. [PubMed] [Google Scholar]
- 5.Spivak H, Schlasinger MH, Tabanda-Lichauco R, Ferstenberg H. Small bowel obstruction from gastrointestinal histoplasmosis in acquired immune deficiency syndrome. Am Surg. 1996;62:369–72. [PubMed] [Google Scholar]
- 6.Lee JT, Dixon MR, Murrell Z, Konyalian V, Agbunag R, Rostami S, et al. Colonic histoplasmosis presenting as colon cancer in the non-immunocompromised patient: Report of a case and review of literature. Am Surg. 2004;70:959–63. [PubMed] [Google Scholar]
- 7.Naniwadekar A, Malhotra A. Education and imaging. Gastrointestinal: Gastrointestinal histoplasmosis. J Gastroenterol Hepatol. 2008;23:668. doi: 10.1111/j.1440-1746.2008.05371.x. [DOI] [PubMed] [Google Scholar]
- 8.Hertan H, Nair S, Arguello P. Progressive gastrointestinal histoplasmosis leading to colonic obstruction two years after initial presentation. Am J Gastroenterol. 2001;96:221–2. doi: 10.1111/j.1572-0241.2001.03479.x. [DOI] [PubMed] [Google Scholar]


