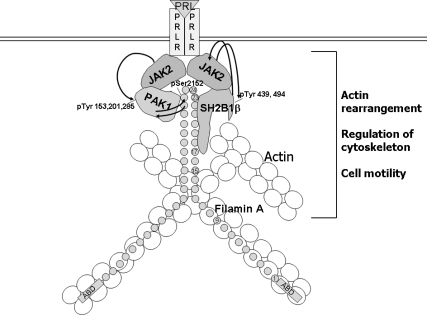
Fig. 12.
PRL-activated JAK2 promotes formation of multiprotein complex. Schematic representation of the proposed working model. PRL-activation of JAK2 leads to tyrosyl phosphorylation of PAK1 on tyrosines 153, 201, and 285, thereby increasing PAK1 activities (both the serine/threonine kinase activity and ability to create potential protein-protein interactions) and stimulating phosphorylation of FLNa. Phosphorylated FLNa stimulates the kinase activity of PAK1 and has increased actin-regulating activity. FLNa, which directly binds to SH2B1β, relocates SH2B1β to the JAK2-PAK1-FLNa complex. Because SH2B1β is the enhancer of the kinase activity of JAK2, the formation of the complex results in enhancement of JAK2 activation and further activation of the JAK2-PAK1-FLNa complex that leads to actin cytoskeleton reorganization via actin-regulating proteins PAK1, FLNa, and SH2B1β. Binding of FLNa to the plasma membrane is not shown.