Fig. 7.
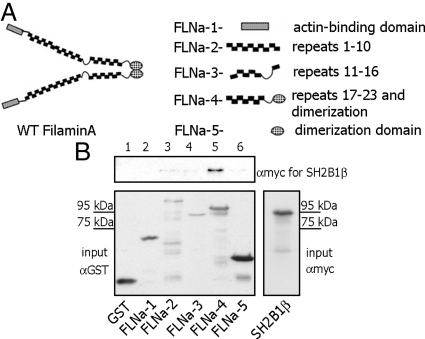
FLNa repeats 17–23 is the site for SH2B1β interaction. A, Schematic depiction of FLNa truncations [modified from Cukier et al. (49)]. B, Different GST-tagged truncated FLNa mutants were purified from bacterial lysates and immobilized on glutathione-agarose beads. Myc-tagged SH2B1β was translated in vitro using TNT Coupled Reticulocyte Lysate System. The mixture of GST-tagged FLNa mutants and in vitro translated SH2B1β was rotated for 1 h. The glutathione-agarose beads were washed, GST-FLNa constructs-bound myc-SH2B1β was detected by IB with αmyc (upper panel). SH2B1β was detected only in lane 5, indicating that only FLNa-4 construct (repeats 17–23 and dimerization domain) directly binds to SH2B1β. Amount of GST or GST-FLNa mutants input was detected by IB with αGST (left bottom panel). Migration of myc- SH2B1β alone is shown on the bottom right panel. Each experiment was performed at least three times with similar results. Lane 1, GST; lane 2, FLNa-1 (actin-binding domain of FLNa); lane 3, FLNa-2 (repeats 1–10); lane 4, FLNa-3 (repeats 11–16); lane 5, FLNa-4 (repeats 17–23 and dimerization domain); and lane 6, FLNa-5 (dimerization domain).