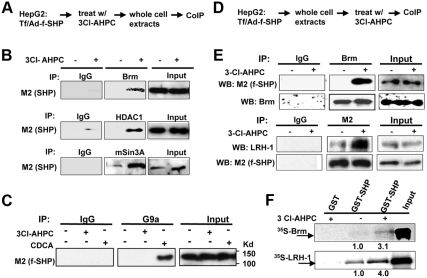
Fig. 3.
3Cl-AHPC increases SHP interaction with LRH-1 and repressive cofactors of SHP, Brm, HDAC1, and mSin3A. A, Experimental outlines. B and C, HepG2 cells were treated with 200 nm 3Cl-AHPC or 50 μm chenodeoxycholic acid for 2 h, and Brm, HDAC1, mSin3A, and G9a were immunoprecipitated from HepG2 cell extracts. Flag-SHP in the immunoprecipitates was detected by Western blot analysis using M2 antibody. D, Experimental outlines. E, HepG2 cell extracts were prepared and then treated with 1 μm 3Cl-AHPC in vitro for 30 min on ice to avoid the activation of cellular kinase signaling. Brm was immunoprecipitated from cell extracts, and the presence of flag-SHP was detected by M2 antibody (top). Flag-SHP was immunoprecipitated from cell extracts and the presence of LRH-1 was detected by Western blot analysis (bottom). In all CoIP assays, IgG was used as a negative control. F, GST-SHP or control GST, which had been expressed in bacteria BL21(DE3) and purified by Glutathione Sepharose, was incubated with 1 μm 3Cl-AHPC or vehicle for 30 min on ice and then further incubated with 35S-Brm or LRH-1 synthesized by in vitro transcriptional and translation in reticulocyte lysates. Interaction of GST-SHP with Brm or LRH-1 was detected by autoradiography. Similar amounts of GST-SHP were used in each reaction (data not shown). Band intensities were determined using ImageJ, and the values for control samples treated with vehicle were set to 1.