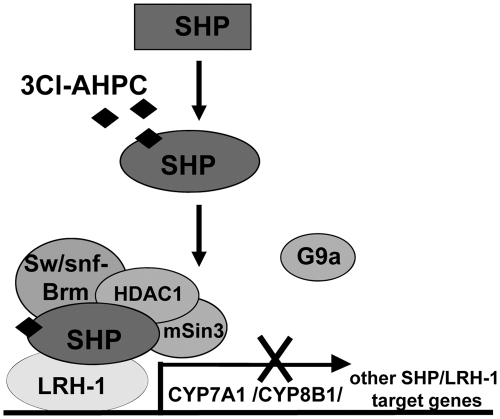
Fig. 8.
Model of the mechanism of the enhancement of SHP activity by 3Cl-AHPC. In this model, 3Cl-AHPC binds to the ligand binding pocket of SHP, which leads to a conformational change of SHP that allows increased interaction of SHP with LRH-1, a DNA binding activator and SHP-docking protein for CYP7A1 and other metabolic genes, such as CYP8B1, SREBP-1c, and ApoA1. Binding of 3Cl-AHPC also selectively increases interaction of SHP with chromatin modifying repressive cofactors, Brm, an ATPase of Swi/Snf chromatin remodeling complexes, and mSin3A/HDAC1 corepressors, but not with G9a lysine methyltransferase. Increased interaction between SHP and LRH-1 leads to increased occupancy of SHP, followed by increased occupancy of repressive cofactors of SHP and decreased occupancy of RNA polymerase II at the promoter of the CYP7A1 and CYP8B1 genes (and possibly other SHP/LRH-1 target genes, such as ApoA1 and SREBP-1c genes), resulting in gene repression. In contrast to this model for nanomolar concentrations of 3Cl-AHPC, micromolar concentrations results in increased CYP7A1 gene expression due to activation of the cellular p38 kinase.