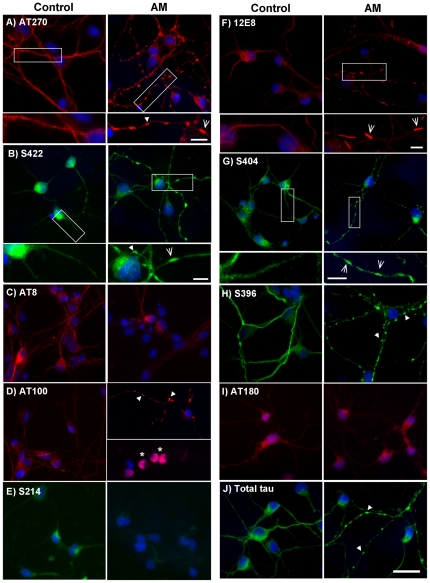
Figure 2. Tau epitopes redistribute in neurons during ATP reduction.
Cells were treated with 1 µM AM for 15 min, fixed and permeabilized for 90 sec with 80% methanol or 0.05% Triton-X. Immunostaining was carried out with antibodies against phosphorylated tau epitopes, as indicated. Staining for total tau and all phospho-epitopes was predominantly smooth and evenly distributed in control cells. Following ATP reduction, distribution of AT8 (C), S214 (E) and AT180 (I) epitope labels remained relatively smooth, although staining intensity was reduced. By contrast, AT270 (A), S422 (B), AT100 (D), 12E8 (F), S404 (G) and S396 (H) labeled accumulations that were mostly spheroid in shape, but occasionally rod-like, following ATP-reduction and were frequently observed in tandem arrays along neurites (arrows). Higher magnification insets of controls vs. AM-treated cells are shown for A, B, F, and G. Correspondingly, some spheroid-like accumulation of total tau (J, arrows) was also evident in neurites of AM-treated cells. Interestingly, only aggregates labeled with 12E8 (F) consistently showed classic and distinct rod-shaped structure throughout AM-treated cells, which is better observed at higher magnification (inset in F, arrows). Additionally, ATP reduction led to the redistribution and nuclear-accumulation of AT100 label (D, arrows), a phenomenon that was observed neither in control cultures stained with AT100, nor in treated cells labeled with any other MAP/tau phospho-epitope. However, nuclear AT100 label is unlikely to represent tau (see text). Nuclei were labeled with DAPI (blue). While most epitopes (except for 12E8 – see Fig. 3) appeared identical under methanol and Triton X permeabilization conditions, images in this figure represent a combination of both protocols. Methanol: (A, B, E, G, J). Triton X: (C, D, F, H, I. Scale bar = 20 µm for all images except insets in (A, B, F, and G) = 5 µm.