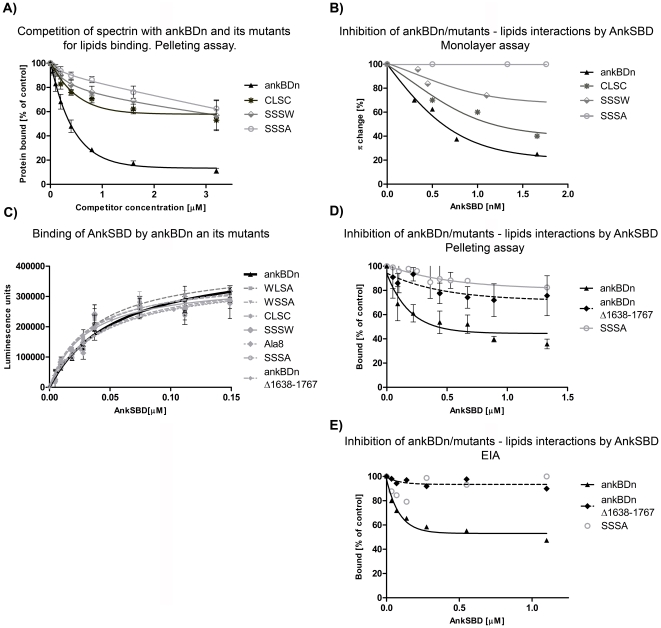
Figure 5. Inhibition assays and binding of ankSBD to ankBDn and its mutants.
A) Inhibition of spectrin-PE/PC liposome binding by ankBDn and its mutants. Fluorescently labeled erythrocyte spectrin (50 nM) was incubated with FAT PE/PC liposomes in the test buffer (see Materials and Methods) at room temperature in the absence (100% binding) or presence of the indicated concentrations of recombinant proteins. B) Inhibition of binding of ankBDn and its mutants to PE/PC monolayers by ankSBD. Recombinant ankBDn or its mutants were preincubated in appropriate proportions with the recombinant ankSBD to obtain the indicated concentrations of ankyrin protein and concentrations of spectrin proteins inducing the Δπ changes below the plateau value. The data points represent average values of three independent experiments with an average variation<10%. C) Binding of ankSBD to ankBDn and its mutants. Data obtained by EIA assay indicate similar affinities of all studied proteins for binding ankSBD. KD values were in the range 27–55 nM. D) Pelleting assay results of inhibition of binding of ankBDn and its mutants to PE/PC liposomes by ankSBD. Recombinant, fluorescently labeled ankBDn or its mutants were preincubated with FAT PE/PC liposomes at room temperature in the absence (100% binding) or presence of the recombinant ankSBD at indicated concentrations. E) EIA results of inhibition of binding of ankBDn and its mutants to PE/PC liposomes by ankSBD.