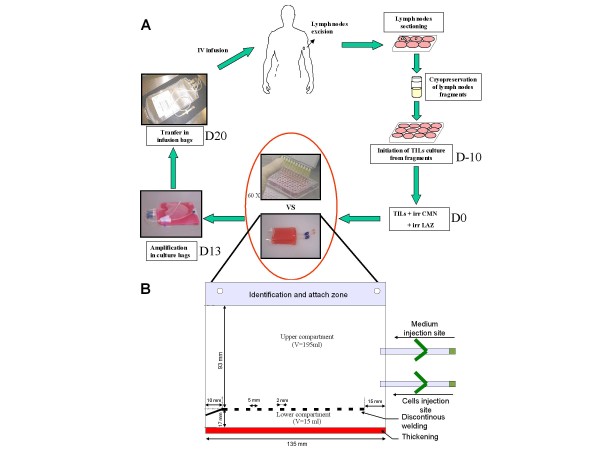
Figure 1.
TIL amplification after stimulation in multiple 96-wells plates or in bags. A, Illustration of the TIL production process for ACT. Briefly, lymph nodes are excised from patients and sectioned for cryopreservation. If the patient is included in the protocol, node fragments are thawed and TIL cultured in 12-well plates for 10 to 14 days. Then, TIL together with feeder cells (irradiated PBMC and irradiated LAZ cells) are plated in sixty 96-well plates for stimulation. Ten days later, TIL are pooled and expanded in culture bags before being administered to patients (see "Materials and Methods" for details). Even though 96-well plates have the advantage of enhancing TIL stimulation through close contact between TIL and feeders cells, they represents a time- and labour-intensive step in the TIL production process. Furthermore, as it requires multiple containers (i.e. sixty 96-well plates), this method is a source of potential contamination and variability of the cell therapy product. In order to make TIL stimulation with feeder cells safer and more straightforward, we developed a specific compartmentalised bag. B, Schematic representation of the compartmentalized bag. This bag is composed of a size-reduced lower compartment (L, 135 mm × H, 17 mm; Vmax 15 ml) that allows close contact between cells. This compartment has been thermoformed to avoid plastic sticking and facilitate cell injection. TIL and feeder cells are injected into this compartment through the bottom injection site. Then culture medium is added in the larger upper compartment (L, 135 mm × H, 93 mm; Vmax 195 ml) via the upper injection site. The two compartments are separated by a discontinuous welding (forteen 2 mm weldings separated by 5 mm) that allows exchanges between both compartments but prevents the passage of cells from the lower to the upper compartment during culture medium renewal.