Abstract
Objective
The goal of the investigation was to study if hypoxia and HIF proteins regulate expression of GlcAT-I, a key enzyme in GAG synthesis in nucleus pulposus (NP) cells.
Methods
QRT-PCR and Western blot were used to measure GlcAT-I expression. Transfections were performed to determine the effect of HIF-1/-2 on GlcAT-I promoter activity.
Results
In hypoxia there was an increase in GlcAT-I expression; a significant increase in promoter activity was seen in both NP and N1511 chondrocytes. We investigated if HIF controlled GlcAT-I expression. Suppression of HIF-1α and HIF-2α induced GlcAT-I promoter activity and expression only in NP cells. While, GlcAT-I promoter activity was suppressed by co-expression of both CA-HIF-1α and CA-HIF-2α only in NP cells suggesting a cell type specific regulation. Site directed mutagenesis and deletion constructs were used to further confirm the suppressive role of HIFs on GlcAT-I promoter function in NP cells. Although it was evident that interaction of HIF with HRE results in suppression of basal promoter activity, it was not necessary for transcriptional suppression. This result suggested both a direct and an indirect mode of regulation possibly through recruitment of a HIF-dependent repressor. Finally we show that hypoxic expression of GlcAT-I was also partially dependent on MAPK signaling.
Conclusions
Results of these studies demonstrate that hypoxia regulates GlcAT-I expression through a signaling network comprising both an activator and suppressor molecules and that this regulation is unique to NP cells.
Keywords: Nucleus pulposus, GlcAT-I, Hypoxia, HIF, GAG synthesis, MAPK
Introduction
The intervertebral disc is a specialized tissue that permits rotation as well as flexure and extension of the human spine. It consists of an outer ligamentous annulus fibrosus that encloses gel-like nucleus pulposus. The superior and inferior boundaries of the intervertebral disc are formed by the cartilage endplates. Blood vessels infiltrate the superficial region of the endplates and the outer third of annulus fibrosus, but do not enter the nucleus pulposus (1, 2). In line with the avascular nature of this tissue there is a robust and constitutive expression of both HIF-1α and HIF-2α suggesting that the nucleus pulposus cells reside in an hypoxic environment (3–5).
The hypoxic cells in the nucleus pulposus secrete a complex extracellular matrix that contains the hydrophilic proteoglycan aggrecan substituted with many glaycosaminoglycan (GAG) chains (6). The major GAG of the nucleus pulposus is chondroitin sulfate. Structurally, this molecule is a heteropolysaccharide containing repeating units of N-acetyl galactosoamine linked to glucuronic acid. In its fully sulfated form, the molecule exhibits a high negative charge density and when hydrated assumes a linear configuration. Bound to the aggrecan core protein, and associated with hyaluronic acid, the chondroitin sulfate chains form a giant polydispersed supramolecular structure. The high osmotic pressure of the aggregate contains the biomechanical forces applied to the spine (7). Surprisingly, while the importance of proteoglycan secretion and function has been discussed by many investigators, mechanisms of control of GAG synthesis, are poorly understood.
A key rate limiting step in chondroitin sulfate synthesis in chondrocytes and possibly nucleus pulposus cells is mediated by galactose–β1,3-glucuronysltransferase-1 (GlcAT-I), the enzyme that catalyzes the transfer of glucuronic acid to the core protein Gal–Gal–Xyl–O-Ser trisaccharide (8–11). Our recent work has shown that in nucleus pulposus cells, GlcAT-I expression is responsive to growth factors, TGFβ and BMP-2 as well as TonEBP, a tonicity sensitive transcription factor (12–14). Previous studies have shown stimulatory effect of hypoxia on aggrecan synthesis in nucleus pulposus cells (15). Whether hypoxia and the HIF family of transcription factors control chondroitin sulfate synthesis in these cells is not known.
The major objective of the investigation was to examine the hypothesis that hypoxia regulates GlcAT-I expression by nucleus pulposus cells. We show for the first time that HIF-1 and HIF-2 serve as negative regulators of GlcAT-I expression in nucleus pulposus cells. Our results suggest that hypoxia and HIF proteins form a regulatory loop that maintains expression of this critical gene in nucleus pulposus cells.
EXPERIMENTAL PROCEDURES
Reagents and Plasmids
Human GlcAT-I reporter plasmids have been reported before (13). pCA-HIF-2α with a triple mutation (P405A/P530A/N851A) (16) were provided by Dr. Celeste Simon, University of Pennsylvania. Plasmids were kindly provided by Dr. Eric Huang, NCI Bethesda [p(HA)HIF-1α(401Δ603) and pARNT], Dr. Melanie Cobb, University of Texas Southwestern Medical Center, Dallas [DN-ERK1 (ERK1K71R) and DN-ERK2 (ERK2K52R)] (17), Robert Freeman, University of Rochester [mouse ShHIF-2α] (18). Rat HIF-2α siRNA and control SiRNA duplexes were purchased from Dharmacon (ON-TARGET plus SMARTpool). SiHIF-1α (PBS/pU6-HIF1α) plasmid developed by Dr. Connie Cepko was obtained from Addgene (#21103) (19). As an internal transfection control, vector pRL-TK (Promega) containing Renilla reniformis luciferase gene was used. The amount of transfected plasmid, the pre-transfection period after seeding, and the post-transfection period before harvesting, have been optimized for rat nucleus pulposus cells using pSV β-galactosidase plasmid (Promega) (4). The N1511 line was a kind gift from Dr. Motomi Enomoto-Iwamoto.
Isolation of nucleus pulposus cells and cell culture in hypoxia
Rat nucleus pulposus cells were isolated using a method reported earlier by Risbud et al. (4). Nucleus pulposus cells were maintained in Dulbeccos Modified Eagles Medium (DMEM) and 10% fetal bovine serum (FBS) supplemented with antibiotics. Nucleus pulposus cells were cultured in an Hypoxia Work Station (Invivo2 300, Ruskinn, UK) with a mixture of 1% O2, 5% CO2 and 94% N2 for 24–72 h. The concentration of oxygen chosen for this study was based on our previous in vitro studies, as well as information generated on the oxemic status of the disc in vivo. In some experiments, N1511 cells, a mouse chondrocyte line maintained in culture as described above was used.
Real time RT-PCR analysis
Following treatment, total RNA was extracted from nucleus pulposus cells using RNAeasy mini columns (Qiagen). Before elution from the column, RNA was treated with RNase free DNAse I. 2 μg of total RNA was used to synthesize cDNA using SuperScipt III cDNA synthesis kit (Invitrogen). Reactions were set up in triplicate in 96 well plate using 1 μl cDNA with SYBR Green PCR Master Mix (Applied Biosystems) to which gene-specific forward and reverse PCR primers were added (GlcAT-I: NCBI # NM_001128184 Fwd: 5′-atgcccagtttgatgctactgcac -3′, Rev: 5′-tgttcctcctgcttcatcttcggt -3′). Each set of samples included a template-free control. PCR reactions were performed in a StepOnePlus real time PCR system (Aplied Biosystems) according to the manufacturer’s instructions. All the primers used were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Immunofluorescence microscopy
Cells were plated in flat bottom 96 well plates (5 × 103/well) and cultured in hypoxia for 24 h. After incubation, cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% triton-X 100 in PBS for 10 min, blocked with PBS containing 5% FBS, and incubated with antibodies against GlcAT-I (1:200) (Novus) at 4 °C overnight. As a negative control, cells were reacted with isotype IgG under similar conditions. After washing, the cells were incubated with Alexa fluor-488 conjugated anti-mouse secondary antibody (Invitrogen), at a dilution of 1:50 and 10 μM propidium iodide for 1 h at room temperature. Cells were imaged using a laser scanning confocal microscope (Olympus Fluoview, Japan).
Protein extraction and Western blotting
Cells were placed on ice immediately following treatment and washed with ice-cold HBSS. Nuclear proteins were prepared using the CellLytic NuCLEAR extraction kit (Sigma-Aldrich, St. Louis). All the wash buffers and final re-suspension buffer included 1X protease inhibitor cocktail (Roche), NaF (5 mM) and Na3VO4 (200 μM). Nuclear or total cell proteins were resolved on 8–12 % SDS-polyacrylamide gels and transferred by electroblotting to PVDF membranes (Bio-Rad, CA). The membranes were blocked with 5% non-fat dry milk in TBST (50 mM Tris, pH 7.6, 150 mM NaCl, 0.1% tween 20) and incubated overnight at 4 °C in 3% non-fat dry milk in TBST with the anti-GlcAT-I (1:500, Novus) or anti-HIF-2α antibody (1:1000, R&D Systems). Immunolabeling was detected using the ECL reagent (Amersham Biosciences).
DMMB assay
The proteoglycan content of the cells cultured for 5 days was measured as sGAG by colorimetric assay with 1–9 dimethylmethylene blue (DMMB) (Blyscan, Biolcolor Ltd., UK) with chondroitin-4-sulphate as a standard following the manufacturer’s instructions. Briefly, GAGs were precipitated from cell extracts and conditioned medium and stained with DMMB and staining was quantified by measuring absorbance at 656 nm. Results were calculated as GAG (μg)/total DNA (ng) and expressed relative to value obtained for untreated controls.
Site directed mutagenesis
GlcAT-I-D reporter plasmid was used to mutate HRE sites (G/ACGTG to G/AAAAG). Mutants were generated using QuickChange II XL site-directed mutagenesis kit (Stratagene), using forward and reverse primer pair containing the desired mutation, following the manufacturer’s instructions. The mutations were verified by sequencing.
Transfections and dual luciferase assay
Cells were transferred to 24-well plates at a density of 4 × 104 cells/well one day before transfection. To investigate the effect of HIF over expression on GlcAT-I promoter activity, cells were cotransfected with 100–300 ng of pSiHIF-1α (100–300 ng) or pSiHIF-2α (100–300 ng) or CA-HIF-1α or CA-HIF-2α (100 ng ARNT with CA-HIF plasmids) or appropriate backbone vector with 300–350 ng GlcAT-I reporter and 300–350 ng pRL-TK plasmid. To measure the effect of hypoxia cells were transfected with 500 ng of GlcAT-I reporter plasmids with 500 ng pRL-TK plasmid, in some experiments nucleus pulposus cells were co-tranfected with SiHIF-2α (25–50 nM) or treated with the inhibitors PD98059 (50 μM), SB203580 (10 μM) and SP60025 (10 μM) (Calbiochem). LipofectAMINE 2000 (Invitrogen) was used as a transfection reagent. For each transfection, plasmids were premixed with the transfection reagent. 48–72 h after transfection, the cells were harvested and a Dual-Luciferase™ reporter assay system (Promega) was used for sequential measurements of firefly and Renilla luciferase activities. Quantification of luciferase activities and calculation of relative ratios were carried out using a luminometer (TD-20/20, Turner Designs, CA). At least three independent transfections were performed, and all analyses were carried out in triplicate.
Statistical analysis
All measurements were performed in triplicate, data is presented as mean ± S.E. Differences between groups were analyzed by the ANOVA; *p < 0.05.
RESULTS
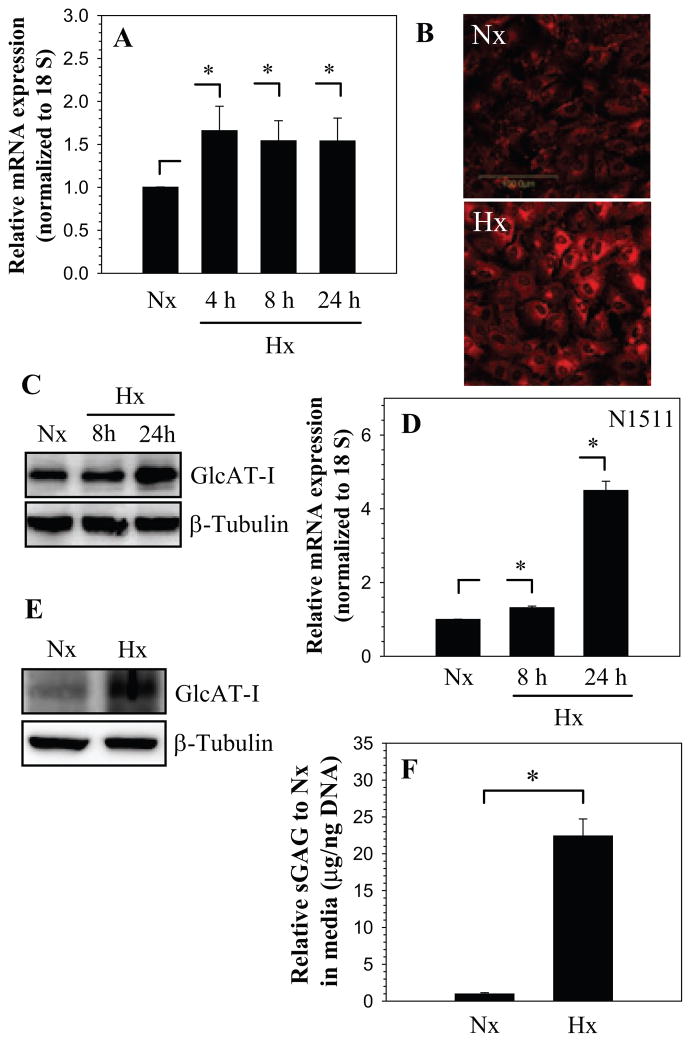
To explore the premise that hypoxia regulated GlcAT-I expression, nucleus pulposus cells were cultured at 1% O2 and the expression of GlcAT-I was analyzed. Figure 1A shows that hypoxia results in increased GlcAT-I mRNA levels in nucleus pulposus cells. In addition, we studied GlcAT-I expression in nucleus pulposus cells using immunofluorescence microscopy and Western blot analysis. Hypoxic treatment results in elevated GlcAT-I protein expression (Fig. 1B, C); the increase is pronounced 24 h after treatment. Similar to nucleus pulposus cells, a control chondrocyte cell line exhibits hypoxic induction in GlcAT-I mRNA and protein levels (Fig. 1D, E). To confirm that the increase in GlcAT-I expression was related to sulfated GAG generation, we measured sGAG in nucleus pulposus cells in hypoxia. Figure 1F shows that compared to normoxia there is a significant induction in sGAG levels in hypoxia.
Figure 1.
A) Real-time RT-PCR analysis of GlcAT-I expression by nucleus pulposus cells cultured in hypoxia (1% O2) up to 24 h. There was increased expression at 4 h which continued till 24 h. B) Immunofluorescent analysis of nucleus pulposus cells in hypoxia. Cells showed increased GlcAT-I expression 24 h after the treatment. C) Western blot analysis of GlcAT-I expression by nucleus pulposus cells. Note, the expression of the 43 kd GlcAT-I band. Note, increased GlcAT-I levels after 24 h in hypoxia. D) Real-time RT-PCR analysis of GlcAT-I expression by N1511 chondrocytes cultured in hypoxia (1% O2) for 24 h. Hypoxia increased expression of GlcAT-I in chondrocytes. E) Western blot analysis showing increased expression of GlcAT-I by N1511 cells in hypoxia. F) Hypoxia significantly increased sGAG production by nucleus pulposus cells. Values shown are mean ± SE, of 3 independent experiments; *p<0.05.
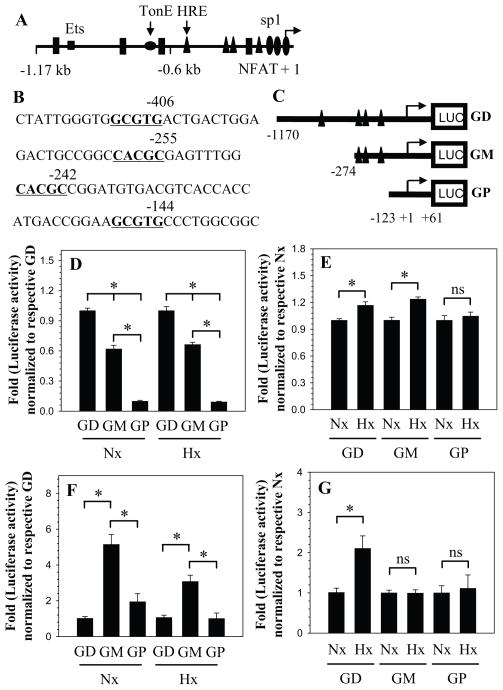
The organization of human GlcAT-I promoter and major transcription factor binding sites are shown in Fig. 2. There are 4 conserved hypoxia response elements (HRE) in the promoter at −406/−411 bp, −255/−260 bp, −242/−247 bp and −144/−149 bp with respect to transcription start site (Fig. 2A, B). To investigate the hypoxic regulation of GlcAT-I transcription, we measured the activity of different size promoter fragments: −1170/+61 bp (GD), a −274/+61 bp (GM) and a −123/+61 bp (GP) (see Fig. 2C) in both nucleus pulposus as well as N1511 chondrocytes. Figure 2D shows that irrespective of oxemic tension GD fragment has maximal basal activity, whereas the GP fragment exhibits the least activity in nucleus pulposus cells. We next examined the effect of hypoxia on activity of these promoter fragments in nucleus pulposus cells. In hypoxia, there is an increase in activity of GD and GM promoter fragment; the activity of shortest promoter fragment GP is unchanged compared to its normoxic activity (Fig. 2E). Unlike the nucleus pulposus cells, in chondrocytes GD fragment exhibits the minimal basal activity while GM fragment showed the highest basal activity in both normoxia and hypoxia (Fig. 2F). Moreover, in N1511 cells while the GD fragment shows hypoxic induction in activity, neither GM and GP fragments are responsive to hypoxia (Fig. 2G).
Figure 2.
A) Schematic of promoter organization of the human GlcAT-I gene showing location of major transcription factor binding sites. The transcription start site is marked as +1. HRE is shown as triangle, TonE as flattened circle, Sp1 are indicted as ovals while the NFAT binding motifs are shown as rectangles. B) Stretches of DNA sequence of the GlcAT-I promoter containing HRE (GCGTG/CACGC) motifs that are marked in bold and underlined. C) Cartoon showing map of successive PCR generated 5′ deletion constructs of the human GlcAT-I promoter. D) Basal activities of GlcAT-I promoter constructs in normoxia and hypoxia relative to full length construct GD in NP cells. E) Hypoxic regulation of GlcAT-I promoter constructs in NP cells. Constructs GD and GM show increase in activity in hypoxia, whereas activity of construct GP is unaffected. F) Relative activities of promoter constructs in N1511 chondrocytes. G) Chondrocytes show hypoxic induction in activity of construct GD, activity of shorter constructs GM and GP is unaffected. Values shown are mean ± SE, of 3 independent experiments; *p<0.05.
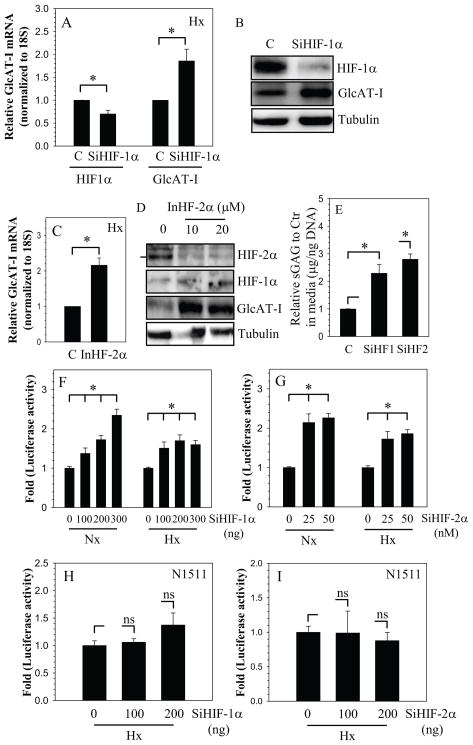
To investigate if HIF-1 plays a role in regulation of GlcAT-I expression in nucleus pulposus cells, we partially silenced HIF-1α expression in nucleus pulposus cells and evaluated GlcAT-I expression in hypoxia. As expected, SiHIF-1α treated cells evidence a decrease in expression of HIF-1α mRNA (Fig. 3A) and protein (Fig. 3B), when compared with cells transfected with control SiRNA. We also confirmed that suppression of HIF-1α lowered its known target gene expression (supplementary Fig. 1). A significant suppression of enolase-1 promoter activity, a known HIF-1 target gene in nucleus pulposus cells is seen. GlcAT-I expression is then evaluated in the silenced cells. We found that there is a robust increase in GlcAT-I expression both at the mRNA (Fig. 3A) and protein (Fig. 3B) level in the silenced cells when compared with cells transfected with control SiRNA. In addition, we treated nucleus pulposus cells with highly specific HIF-2α translational inhibitor, methyl-3 (2(cyano(methylsulfonyl)methylene)hydrazino)thiophene-2-carboxylate (20), and measured GlcAT-I expression. Suppression in HIF-2α level resulted in significant increase in GlcAT-I mRNA (Fig. 3C) and protein (Fig. 3D) expression. To investigate if change in HIF and in turn GlcAT-I expression corresponds to change in GAG synthesis, we measured GAG levels in HIF silenced cells. Fig. 3E shows that there is a significant increase in GAG synthesis when expression of both HIF-1α and HIF-2α is suppressed (Fig. 3E).
Figure 3.
HIF regulation of GlcAT-I expression. Cells were transfected with HIF-1α-SiRNA (SiHIF-1α) or control siRNA (C) and cultured in hypoxia. A, B) Partial silencing of HIF-1α results in increased GlcAT-I mRNA (A) and protein (B) levels. C) NP cells treated with HIF-2α translation inhibitor in hypoxia shows induction in GlcAT-I mRNA expression D) Western blot analysis of NP cells treated with HIF-2α inhibitor. Treated cells show a significant decrease in HIF-2α levels, concomitant increase in GlcAT-I expression is evident. E) Silencing of both HIF-1α (SiHF1α) and HIF-2α (SiHF2α)results in increased GAG deposition by NP cells. F, G) Effect of HIF on GlcAT-I promoter activity in NP and N1511 cells. NP cells were transfected with F) SiHIF-1α G) SiHIF-2α and cultured in normoxia (Nx) and hypoxia (Hx). Control cells were transfected with control siRNA (C). Silencing of HIF-1α or HIF-2α resulted in a significant induction of GlcAT-I promoter activity irrespective of oxemic state. H, I) Silencing of HIF-1α (H) and HIF-2α (I) has no effect on GlcAT-I promoter activity in N1511 chondrocytes. Values shown are mean ± SE from three independent experiments, * p < 0.05.
To further evaluate the role of HIF-1 and HIF-2 in transcriptional regulation of GlcAT-I expression, the activity of the −1170/+61 bp promoter fragment was measured by performing loss of function studies. When HIF-1α expression was silenced using siRNA, GlcAT-I promoter activity is induced under both normoxic and hypoxic conditions in nucleus pulposus cells (Fig. 3F). Interestingly, induction of activity is similar in both normoxia and hypoxia. To assess the role of HIF-2α in regulation of GlcAT-I, we co-transfected nucleus pulposus cells with HIF-2α SiRNA. It was noted that HIF-2α silencing results in induction in promoter activity, independent of oxemic status (Fig. 3G). In contrast to nucleus pulposus cells, silencing of either HIF-1α (Fig. 3H) or HIF-2α (Fig. 3I) did not affect GlcAT-I promoter activity in chondrocytes.
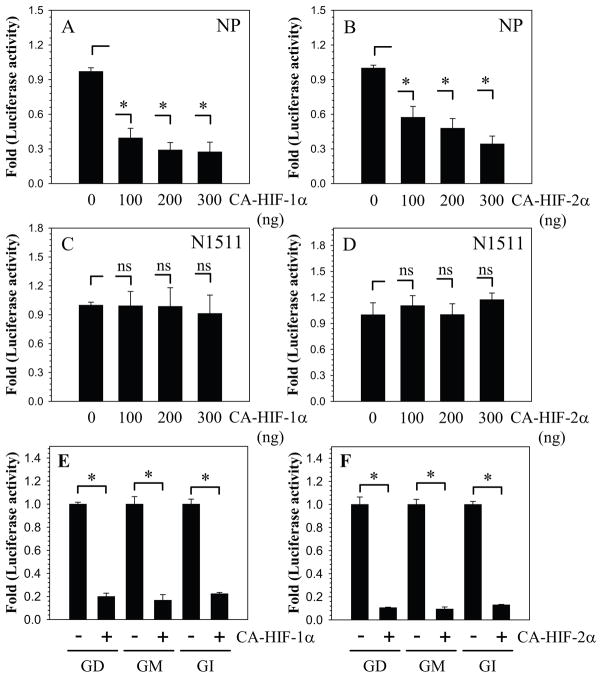
To validate whether GlcAT-I promoter activity is responsive to HIF signaling, we co-transfected nucleus pulposus cells with plasmids encoding CA-HIF-1α or CA-HIF-2α which are insensitive to oxidative degradation (Fig. 4A, B). Transfection with CA-HIF-1α (Fig. 4A) as well as CA-HIF-2α (Fig. 4B) significantly suppresses GlcAT-I promoter (−1170/+61 bp) activity. The inhibitory effect of CA-HIF-1α on GlcAT-I promoter activity is such that when the concentration of plasmid is decreased from 300 to 100 ng level of inhibition remains constant (Fig. 4A). For CA-HIF-2α, while suppression of promoter activity is evident at 100 ng, the effect is further enhanced when the concentration of plasmid is increased to 300 ng (Fig. 4B). As expected, co transfection of CA-HIF-1α or CA-HIF-2α does not affect GlcAT-I promoter activity in N1511 chondrocytes (Fig. 4C, D). To determine the region of the promoter that is responsible for HIF mediated suppression, we measured the activity of the three promoter fragments in the presence of HIF-1α and HIF-2α. It is evident that activity of all three promoter fragments is suppressed by both HIF-1 and HIF-2 (Fig. 4E, F).
Figure 4.
Effect of A, B) CA-HIF-1α and C, D) CA-HIF-2α expression on GlcAT-I promoter activity in nucleus pulposus (A, C) and N1511 (B, D) chondrocytes. Note the significant suppression in GlcAT-I reporter activity even when transfected with 100 ng of the HIF plasmid. In contrast, HIF overexpression has no effect on GlcAT-I promoter activity in N1511 chondrocytes. E, F) Overexpression of HIF-1α and HIF-2α results in suppression of GD, GM as well as GP promoter fragments in nucleus pulposus cells. Values shown are mean ± SE, of 3 independent experiments; *p<0.05, ns = non significant.
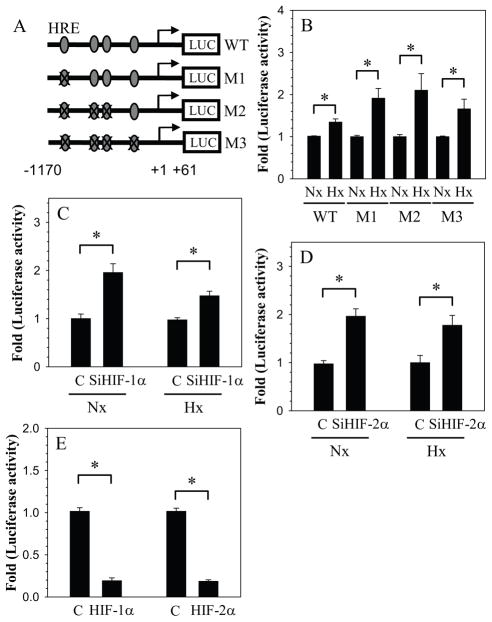
We then examined the interaction of HIFs with HRE in the GlcAT-I promoter. For this study we used reporters that contained mutation in either single (M1) or multiple (M2 and M3) HREs (Fig. 5A). Compared to the wild type reporter, all the mutant reporters are more responsive to hypoxia and showed increased induction in activity (Fig. 5B). We examined the effect of HIF-1α and HIF-2α silencing on M3 reporter activity. The results clearly show that similar to wild type reporter, irrespective of oxemic tension HIF-1α silencing leads to increased activation of M3 reporter (Fig. 5C). A similar trend is seen following HIF-2α silencing (Fig. 5D). Moreover, overexpression of HIF-1α as well as HIF-2α results in suppression of activity of the mutant reporter (Fig. 5E).
Figure 5.
Interaction of HIFs with HREs in the GlcAT-I promoter. A) Schematic of the GlcAT-I reporter constructs (WT: wild type; M1: HRE1 mutant; M2: HRE1/2/3 mutant, M3:HRE1/2/3/4 mutant) used in transfections. B) Effect of hypoxia on the activity of WT and HRE mutants. HRE mutants show a higher induction in activity in hypoxia compared to wild type reporter. C, D) Effect of silencing of C) HIF-1α (SiHIF-1α) and D) HIF-2α (SiHIF-2α) on mutant reporter M3 in normoxia (Nx) and hypoxia (Hx). Silencing of both HIF-1α and HIF-2α caused induction in the activity of HRE mutant M3 that is independent of O2 tension. E) Effect of CA-HIF-1α and CA-HIF-2α co-expression on M3 mutant activity. Both HIF-1α and HIF-2α suppressed the activity of M3. Values shown are mean ± SE from three independent experiments, * p < 0.05; ns = non significant.
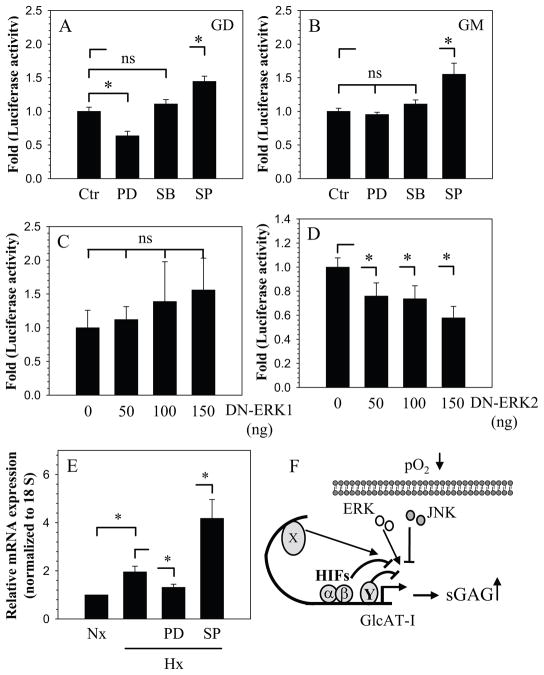
We next investigated the involvement of the MAPK signaling pathway in hypoxic induction of GlcAT-I. Cells were treated with PD98059 (ERK inhibitor) or SB203580 (p38 inhibitor) in hypoxia and promoter activity was measured. Figure 6A shows that ERK but not p38 inhibition suppresses the hypoxic activity of the −1170/+61 bp GlcAT-I (GD) promoter fragment. However, the activity of the −274/+61 bp (GM) promoter fragment is insensitive to both ERK and p38 inhibition (Fig. 6B). In contrast to ERK and p38, inhibition of JNK results in hypoxic induction of both the promoter fragments (Fig. 6A, B). To investigate which isoform of ERK was responsible for the regulation, we co-transfected cells with DN-ERK1 and DN-ERK2 plasmids. Figure 6C, D show that inhibition of ERK2 but not ERK1 results in suppression of GlcAT-I (−1170/+61 bp) promoter activity in hypoxia. To validate role of MAPK in regulation of GlcAT-I expression in hypoxia, we measured mRNA expression following treatment with inhibitors. Figure 6E shows that treatment with PD98059 suppressed GlcAT-I expression while JNK inhibition results in further increase in expression.
Figure 6.
NP cells were transfected with GlcAT-I reporter plasmids GD (A) and GM (B) and cultured with or without MAPK inhibitors PD98059 (PD; 50 μM), SB86002 (SKF; 10 μM) and SP600125 (SP; 10 μM) in hypoxia for 24 h. PD caused suppression of only GD, while no change in activity was observed when treated with SB. In contrast, SP promoted the hypoxic activity of both GD and GM reporters. C, D) Cells were co-transfected with either DN-ERK1 (C) or DN-ERK2 (D) with reporter GD. DN-ERK2, but not DN-ERK1, resulted in suppression of reporter. E) NP cells were treated with MAPK inhibitors in hypoxia and GlcAT-I mRNA expression analyzed. Expression decreased with PD treatment while SP induced expression. Values shown are of 3 independent experiments; mean ± SE; *p < 0.05. F) A proposed model of GlcAT-I regulation by hypoxia and HIF in NP cells. In hypoxia, GlcAT-I level is maintained by transcriptional induction through yet unknown factor (X). On the other hand, HIF-1 and HIF-2 serve as transcriptional suppressor, directly by interaction with HRE and indirectly through an unknown repressor Y. Stimulatory effect of hypoxia dominates repression through HIFs resulting in net increase in GlcAT-I levels and increased GAG production.
Based on these experiments a working model of GlcAT-I regulation by hypoxia and HIF transcription factors in nucleus pulposus cells is proposed (Fig. 6E)
DISCUSSION
The goal of this investigation was to test the hypothesis that the expression of GlcAT-I, a key enzyme required for the biosynthesis of chondroitin and heparan sulfate is regulated by HIF. The hypothesis was based on previous studies that showed that there was significant expression of GlcAT-I in the nucleus pulposus, a tissue that is devoid of vasculature (12, 13). The study revealed that there was a hypoxia-dependent increase in GlcAT-I expression. On the other hand, HIF negatively regulated its expression. Use of deletion constructs together with gain and loss of function studies indicated that HIF directly suppressed basal GlcAT-I promoter activity through interactions with one or more HREs, although it was not required for transcriptional suppression. We put forward the hypothesis that suppression is mediated directly through binding to the HREs and indirectly by an as yet undefined repressor molecule (Fig. 6F). Hence, within the hypoxic environment of the intervertebral disc, members of the HIF transcription factor family and MAPK regulate GlcAT-I expression through an activator - repressor signaling circuit.
We observed that GlcAT-I expression was inducible by hypoxiain both nucleus pulposus cell and a chondrocyte line. These findings are in line with a report by Robins et al. which showed an increased hypoxic expression of GlcAT-I in stromal cells (21). Studies with promoter deletion constructs indicated significant differences in basal activity between nucleus pulposus cells and chondrocytes. This finding suggested that promoter regulation was cell type specific. In the same study, we determined if the conserved HRE sites played a regulatory role in mediating the hypoxic response. Again, the response varied, lending strength to the notion that regulation is unique to nucleus pulposus cells.
With respect to hypoxia, there is one overriding characteristic of HIF-1α and HIF-2α expression in nucleus pulposus cells: expression is refractory to oxemic status (3–5). However, gain and loss of function studies suggested that in nucleus pulposus cells, GlcAT-I expression is regulated in a HIF-dependent manner and that HIF functions as a transcriptional repressor of GlcAT-I. This result is in line with the recent report that HIF proteins serve as transcriptional repressors in nucleus pulposus cells (22) and that HIF-1 and HIF-2 play a more complimentary role in nucleus pulposus cells than has been reported in articular cartilage (5, 22). Once again, these responses were markedly different from those observed using the chondrocytic N1511 cell line lending support to the notion that there is a unique relationship between HIF and GlcAT-I expression in nucleus pulposus cells.
The over expression studies provided further details on the possible repressor role of HIF. In nucleus pulposus cells, HIF-1α or HIF-2α over expression suppressed the activity of both the −274/+61 and −127/+61 promoter fragments that contained either two proximal or no HRE motif respectively. This finding suggests that in nucleus pulposus cells, a HIF-dependent repressor is likely recruited to the promoter sequence between bases −127/+61; in this case, direct binding of HIF is not a pre-requisite for activity suppression (see Fig. 5). Site directed mutagenesis studies confirmed this observation. Thus, the increase in mutant promoter activity in hypoxia suggested that direct binding of HIF to HREs was a partial cause of the observed repression. Indeed, subsequent studies showed that the activity of the HRE mutant reporter was sensitive to modulation of HIF levels. Accordingly, these loss and gain of function experiments strongly suggested that HIF controlled GlcAT-I promoter activity both by direct interaction with HREs and indirectly by recruitment of another repressor. Moreover, it was apparent that direct binding of HIF to the HREs was not necessary for the suppression. Nevertheless, we conclude that in the hypoxic intervertebral disc, the expression of HIF-1 and HIF-2 serve to control basal GlcAT-I expression. Further studies are currently underway in our laboratory to determine the identity of HIF-dependent repressor involved in regulation of GlcAT-I expression in cells of the nucleus pulposus.
To delineate signaling mechanisms that transduce hypoxic expression of GlcAT-I in nucleus pulposus cells, we focused first on the MAPK pathways using loss of function approach. In earlier studies, we noted the significance of this signaling pathway in regulating the functional and survival activities of cells of both the nucleus pulposus and annulus fibrosus (23–25). Moreover, we have recently shown that in nucleus pulposus cells, MAPK mediates TGFβ and BMP-2 dependent increase in GlcAT-I promoter activity as well glycosoaminoaglycan accumulation (12). Similarly, Barré et al. reported that calcium ions promoted GlcAT-I transcription by activation and recruitment of Sp1 to the promoter through MEK/ERK signaling (26). Thus, the importance of the MAPK signaling pathway in mediating nucleus pulposus cell function is well documented (23–25). Related to the expression of GlcAT-I, we found that if ERK is inhibited pharmacologically, there is suppression of hypoxic GlcAT-I reporter activity. Moreover unlike ERK1 which plays a role in growth factor mediated GlcAT-I regulation, in hypoxia, the ERK2 isoform was required for GlcAT-I promoter activity (12). We interpreted this finding to indicate that these MAPK isoforms were not redundant, but were modulated by, and responsive to, separate physiological stimuli.
Deletion analysis indicated that the region between −1170 bp to −274 bp of the GlcAT-I promoter was responsible for ERK regulation. Since this region is a distance from Sp1 site (see schematic, Fig. 2), it precludes the possibility that this motif was responsible for hypoxic induction of GlcAT-I promoter activity in nucleus pulposus cells (13, 24). Interestingly, unlike ERK, suppression of JNK resulted in further induction of both the −1170 bp and −274 bp fragments of GlcAT-I promoter in hypoxia. Whether JNK interacts with a HIF responsive repressor has yet to be determined. However, this result, supported by the mutagenesis and deletion analysis, indicates that a strong repressive element is present within first 127 bases and is responsible for regulating hypoxic expression of GlcAT-I. Future studies are aimed at investigating this hypothesis and the exact nature of the repressor.
Supplementary Material
Acknowledgments
This study was supported by NIH grants # AR050087 and AR055655
This work was supported by grants from the National Institutes of Health R01-AR050087 and R01-AR055655.
Contributor Information
Shilpa S. Gogate, Email: shilpa.gogate@jefferson.edu.
Rena Nasser, Email: rena.nasser@jefferson.edu.
Irving M. Shapiro, Email: irving.shapiro@jefferson.edu.
Makarand V. Risbud, Email: makarand.risbud@jefferson.edu.
References
- 1.Hassler O. The human intervertebral disc. A micro-angiographical study on its vascular supply at various ages. Acta Orthop Scand. 1969;40:765–772. doi: 10.3109/17453676908989540. [DOI] [PubMed] [Google Scholar]
- 2.Rudert M, Tillmann B. Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop Scand. 1993;64:37–40. doi: 10.3109/17453679308994524. [DOI] [PubMed] [Google Scholar]
- 3.Rajpurohit R, Risbud MV, Ducheyne P, Vresilovic EJ, Shapiro IM. Phenotypic characteristics of the nucleus pulposus: expression of hypoxia inducing factor-1, glucose transporter-1 and MMP-2. Cell Tissue Res. 2002;308:401–407. doi: 10.1007/s00441-002-0563-6. [DOI] [PubMed] [Google Scholar]
- 4.Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, Schaer TP, Albert TJ, Shapiro IM. Nucleus pulposus cells express HIF-1alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal A, Gajghate S, Smith H, Anderson DG, Albert TJ, Shapiro IM, Risbud MV. Cited2 modulates hypoxia-inducible factor-dependent expression of vascular endothelial growth factor in nucleus pulposus cells of the rat intervertebral disc. Arthritis Rheum. 2008;58:3798–808. doi: 10.1002/art.24073. [DOI] [PubMed] [Google Scholar]
- 6.Feng H, Danfelter M, Strömqvist B, Heinegård D. Extracellular matrix in disc degeneration. J Bone Joint Surg Am. 2006;88:25–29. doi: 10.2106/JBJS.E.01341. [DOI] [PubMed] [Google Scholar]
- 7.Ng L, Grodzinsky AJ, Patwari P, Sandy J, Plaas A, Ortiz C. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. J Struct Biol. 2003;143:242–257. doi: 10.1016/j.jsb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Kitagawa H, Ujikawa M, Sugahara K. Developmental changes in serum UDP-GlcA:chondroitin glucuronyltransferase activity. J Biol Chem. 1996;271:6583–6585. doi: 10.1074/jbc.271.12.6583. [DOI] [PubMed] [Google Scholar]
- 9.Venkatesan N, Barré L, Benani A, Netter P, Magdalou J, Fournel-Gigleux S, Ouzzine M. Stimulation of proteoglycan synthesis by glucuronosyltransferase-I gene delivery: a strategy to promote cartilage repair. Proc Natl Acad Sci U S A. 2004;101:18087–18092. doi: 10.1073/pnas.0404504102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai X, Wei G, Sinha A, Esko JD. Chinese hamster ovary cell mutants defective in glycosaminoglycan assembly and glucuronosyltransferase I. J Biol Chem. 1999;274:13017–13024. doi: 10.1074/jbc.274.19.13017. [DOI] [PubMed] [Google Scholar]
- 11.Gouze JN, Bordji K, Gulberti S, Terlain B, Netter P, Magdalou J, Fournel-Gigleux S, Ouzzine M. Interleukin-1beta down-regulates the expression of glucuronosyltransferase I, a key enzyme priming glycosaminoglycan biosynthesis: influence of glucosamine on interleukin-1beta-mediated effects in rat chondrocytes. Arthritis Rheum. 2001;44:351–360. doi: 10.1002/1529-0131(200102)44:2<351::AID-ANR53>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Hiyama A, Gogate SS, Gajghate S, Mochida J, Shapiro IM, Risbud MV. BMP-2 and TGF-beta stimulate expression of beta1,3-glucuronosyl transferase 1 (GlcAT-1) in nucleus pulposus cells through AP1, TonEBP, and Sp1: role of MAPKs. J Bone Miner Res. 2010;25:1179–1190. doi: 10.1359/jbmr.091202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiyama A, Gajghate S, Sakai D, Mochida J, Shapiro IM, Risbud MV. Activation of TonEBP by calcium controls β1,3-glucuronosyltransferase-I expression, a key regulator of glycosaminoglycan synthesis in cells of the intervertebral disc. J Biol Chem. 2009;284:9824–9834. doi: 10.1074/jbc.M807081200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai TT, Danielson KG, Guttapalli A, Oguz E, Albert TJ, Shapiro IM, Risbud MV. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem. 2006;281:25416–25424. doi: 10.1074/jbc.M601969200. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal A, Guttapalli A, Narayan S, Albert TJ, Shapiro IM, Risbud MV. Normoxic stabilization of HIF-1alpha drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007;293:C621–31. doi: 10.1152/ajpcell.00538.2006. [DOI] [PubMed] [Google Scholar]
- 16.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell. 2007;18:4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson MJ, Harkins PC, Zhang J, Baer R, Haycock JW, Cobb MH, Goldsmith EJ. Mutation of position 52 in ERK2 creates a nonproductive binding mode for adenosine 5′-triphosphate. Biochemistry. 1996;35:5641–5646. doi: 10.1021/bi952723e. [DOI] [PubMed] [Google Scholar]
- 18.Lomb DJ, Desouza LA, Franklin JL, Freeman RS. Prolyl hydroxylase inhibitors depend on extracellular glucose and hypoxia-inducible factor (HIF)-2alpha to inhibit cell death caused by nerve growth factor (NGF) deprivation: evidence that HIF-2alpha has a role in NGF-promoted survival of sympathetic neurons. Mol Pharmacol. 2009;75:1198–209. doi: 10.1124/mol.108.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen B, Cepko CL. HDAC4 regulates neuronal survival in normal and diseased retinas. Science. 2009;323:256–259. doi: 10.1126/science.1166226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmer M, Ebert BL, Neil C, Brenner K, Papaioannou I, Melas A, Tolliday N, Lamb J, Pantopoulos K, Golub T, Iliopoulos O. Small-molecule inhibitors of HIF-2a translation link its 5′UTR iron-responsive element to oxygen sensing. Mol Cell. 2008;32:838–48. doi: 10.1016/j.molcel.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, Clemens TL. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 22.Skubutyte R, Markova D, Freeman TA, Anderson DG, Dion AS, Williams CJ, Shapiro IM, Risbud MV. HIF regulation of ANK expression in nucleus pulposus cells: Possible implications in controlling dystrophic mineralization in the intervertebral disc. Arthritis Rheum. 2010;62:2707–2715. doi: 10.1002/art.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Risbud MV, Fertala J, Vresilovic EJ, Albert TJ, Shapiro IM. Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions and resist apoptosis induced by serum withdrawal. Spine. 2005;30:882–889. doi: 10.1097/01.brs.0000159096.11248.6d. [DOI] [PubMed] [Google Scholar]
- 24.Risbud MV, Di Martino A, Guttapalli A, Seghatoleslami R, Denaro V, Vaccaro AR, Albert TJ, Shapiro IM. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and annulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine. 2006;31:884–890. doi: 10.1097/01.brs.0000209335.57767.b5. [DOI] [PubMed] [Google Scholar]
- 25.Uchiyama Y, Cheng CC, Danielson KG, Mochida J, Albert TJ, Shapiro IM, Risbud MV. Expression of acid-sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc is regulated by p75NTR and ERK signaling. J Bone Miner Res. 2007;22:1996–2006. doi: 10.1359/jbmr.070805. [DOI] [PubMed] [Google Scholar]
- 26.Barré L, Venkatesan N, Magdalou J, Netter P, Fournel-Gigleux S, Ouzzine M. Evidence of calcium-dependent pathway in the regulation of human beta1,3-glucuronosyltransferase-1 (GlcAT-I) gene expression: a key enzyme in proteoglycan synthesis. FASEB J. 2006;20:1692–1694. doi: 10.1096/fj.05-5073fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.