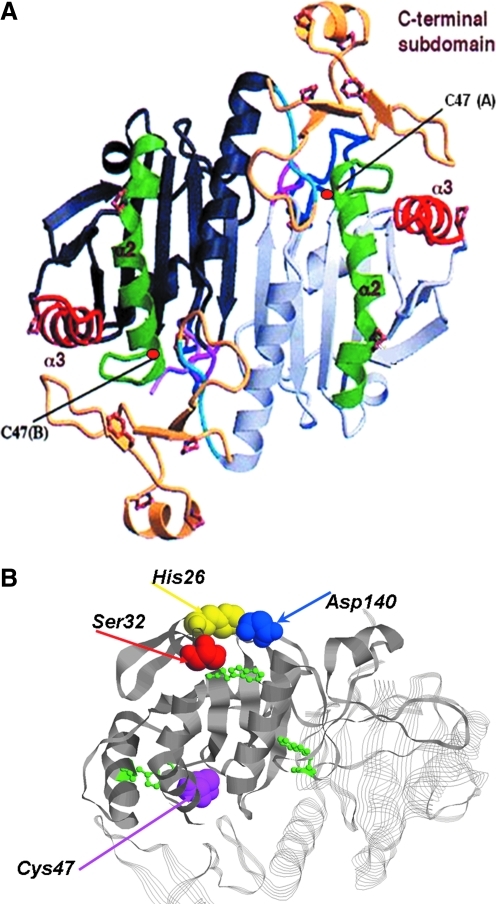
FIG. 2.
Tertiary structure of Prdx6. (A) Crystal structure of mutated (C91S) human Prdx6 that was oxidized by air exposure. The protein crystallized as a homodimer. Notable features are the thioredoxin fold consisting of a four-stranded β sheet with two flanking α helices. The c-terminal domain comprises amino acid residues 175–224 and consists of 3 β strands and an α helix attached to the larger internal domain by a short loop. The catalytic C47 in each monomer is indicated. Modified from Choi et al. (7). (B) Ribbon diagram showing the relative position of the active site for peroxidase activity (C47) and the catalytic triad for phospholipase A2 (PLA2) activity (S32, H26, D140). The SDH catalytic triad is on the protein surface, whereas C47 is at the base of a narrow pocket. Also indicated (in green) are the positions of the three Trp residues that have been used to analyze substrate binding (54, 55). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).