Abstract
Stable major histocompatibility complex (MHC) class I molecules at the cell surface consist of three separate, noncovalently associated components: the class I heavy chain, the β2-microglobulin light chain, and a presented peptide. These three components are assembled inside cells via complex pathways involving many other proteins that have been studied extensively. Correct formation of disulfide bonds in the endoplasmic reticulum is central to this process of MHC class I assembly. For a single specific peptide to be presented at the cell surface for possible immune recognition, between hundreds and thousands of peptide-containing precursor polypeptides are required, so the overall process is relatively inefficient. To increase the efficiency of antigen presentation by MHC class I molecules, and for possible therapeutic purposes, single-chain molecules have been developed in which the three, normally separate components have been joined together via flexible linker sequences in a single polypeptide chain. Remarkably, these single-chain MHC class I molecules fold up correctly, as judged by functional recognition by cells of the immune system, and more recently by X-ray crystallographic structural data. This review focuses on the interesting properties and potential of this new type of engineered MHC class I molecule. Antioxid. Redox Signal. 15, 645–655.
Introduction
Major histocompatibility complex (MHC) class I molecules play a key role in controlling the biological activity of various different cell types of the immune system. By displaying a processed sample of the intracellular contents of a cell at its surface, they allow detection of infection and cellular transformation, and subsequent appropriate effector responses. Even in the absence of any abnormality, the absolute level of expression of MHC class I at the cell surface helps to control the activation threshold of many cell types of the immune system. MHC class I molecules also select the CD8+ T cell repertoire during T cell development in the thymus. Probably the best characterized role for MHC class I molecules is in the generation of CD8+ T cell responses, but MHC class I molecules also play important roles in delivering signals to natural killer (NK) cells, neutrophils, and other myeloid cell types: depending on the context, they can activate or inhibit immune responses. More recently, it has become clear that MHC class I molecules also play a role in shaping the nervous system, at least in mice (10, 19).
The biochemistry and cell biology of MHC class I molecules have been studied extensively for the past 30 years, and we now have a detailed picture of how these molecules fold and are assembled in the endoplasmic reticulum (ER) from their three distinct components: the class I heavy (H) chain, the β2-microglobulin (β2m) light chain, and a presented peptide, usually 8–10 amino acid residues in length (see other reviews in this issue). Nevertheless, there are still aspects of the MHC class I antigen presentation pathway that are not fully understood. MHC class I genes are highly polymorphic, and individuals express multiple alleles. These different MHC class I molecules may have different properties, in terms of their efficiency of assembly, dependence on chaperones, and rate of intracellular transport, and may also influence each others function in specific combinations. Competition between MHC class I molecules has been described (53, 54), but very little is known about this aspect of the class I pathway. The mechanisms involved in the presentation of peptides from internalized antigen by MHC class I molecules, a process known as cross presentation, are also incompletely understood, and form an area of very active investigation (25). In addition, there is continued interest in improving the methods available for generating CD8+ T cell responses in vivo and for expanding CD8+ T cell populations in vitro for possible therapeutic purposes. Enhanced CD8+ T cell responses may be beneficial in a variety of infectious diseases, and also for immunotherapy of tumors.
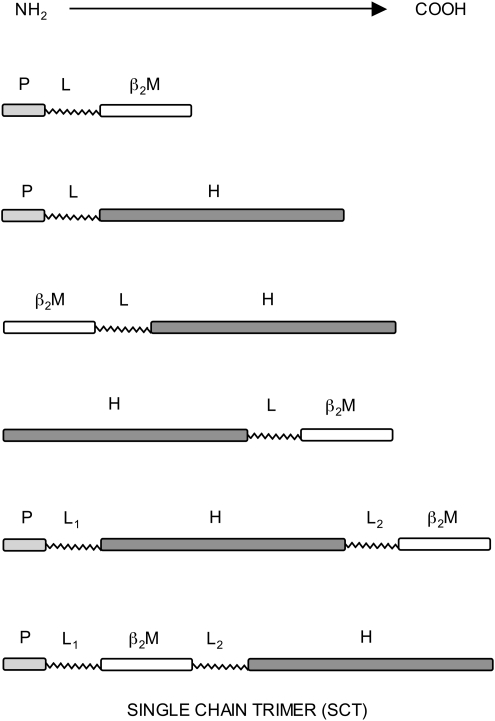
A logical and attractive option for improving the efficiency of assembly of MHC class I molecules, and hence improve their ability to stimulate immune responses, is to covalently link together their normally separate components, and various strategies have been used to do this, since 1991 (38). This approach has the potential to bypass any antigen processing requirements, and may generate MHC class I molecules of increased stability. It may also make class I molecules less dependent on chaperone assistance, and more resistant to downregulation by viruses and in tumors. Figure 1 illustrates schematically the different approaches tested by various groups. Fusions including the class I H chain at the C-terminal end have been generated both with and without a transmembrane anchor, allowing the production of soluble protein. Fusions containing β2m at the C-terminal end necessarily produce soluble protein. For expression in mammalian cells, an N-terminal signal sequence for entry into the ER is required, and that of β2m is normally used. Although initially fusions via a glycine/serine-containing flexible linker sequence were made between two components, either β2m with class I H chain (7, 13, 26, 27, 33, 38, 40, 52, 68), peptide with β2m (51, 57, 58, 61), or peptide with class I H chain (11, 39), more recently it has become clear that fusions between all three components, in the order peptide, linker 1, β2m, linker 2, and class I H chain are optimal (14, 59, 65). This overall organization has proved to be applicable to many different MHC class I allele–peptide combinations from several different species, and this type of construct has become known as a class I single-chain trimer (SCT), as pioneered by Hansen and his colleagues (47, 65). Fusions containing the three components in other orders do not work so well (50). This review will focus on the properties of MHC class I SCTs, their applications so far, and their exciting potential uses.
FIG. 1.
Outline structures of different fusions generated between components of MHC class I molecules. P, peptide epitope; L, flexible linker sequence composed of glycine and serine residues; β2M, β2-microglobulin; H, MHC class I heavy chain; MHC, major histocompatibility complex.
Characterization of MHC Class I SCT Molecules
Although fusions between antigenic peptide and β2m, and between β2m and class I H chain were shown to be functional and are useful reagents under some circumstances, it was only with the development of the SCT format outlined above that it became possible for all the MHC class I molecules expressed in a cell to present the same peptide. Initial reports of SCTs used three different class I H chains: mouse H-2Kk (59), H-2Kb (65), and human leukocyte antigen (HLA)-A2 (14). Functional single-chain MHC class II molecules had already been described (21, 48), but it was perhaps surprising that the class I SCT format appeared to work so well: the linker extending from the C-terminal end of the presented peptide toward β2m would have been expected to disrupt the usual anchoring of the peptide in the H chain F pocket, where the peptide-binding groove is normally closed (32). Nevertheless, the first generation of SCTs was found to maintain their single-chain covalent structure and to be functional, as judged by both T cell and antibody recognition: in addition to class I conformation-specific monoclonal antibodies (mAbs), antibodies with T cell receptor (TCR)-like specificity for a combination of class I complexed with a specific peptide also recognized SCTs (59, 65). Glycine and serine-containing flexible linkers of various lengths and sequences have been used to generate class I SCTs, but Hansen and colleagues reported that a first linker of 15 residues (G4S)3 and a second linker of 20 residues (G4S)4 were optimal (65), although not all possible combinations were tested. We developed class I SCTs independently, and routinely use the amino acid sequences G6(SGG)3 as linker one and (G4S)3 as linker two in SCT constructs, linker sequences based on previously reported two component fusions (33, 51).
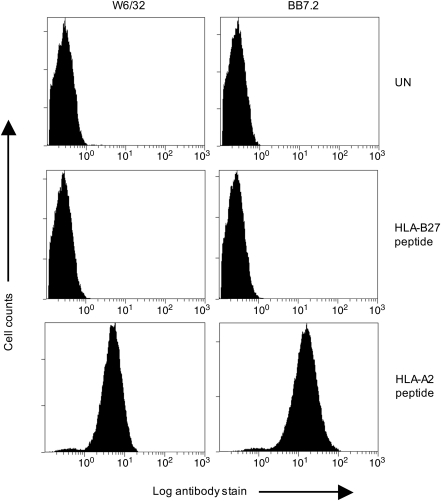
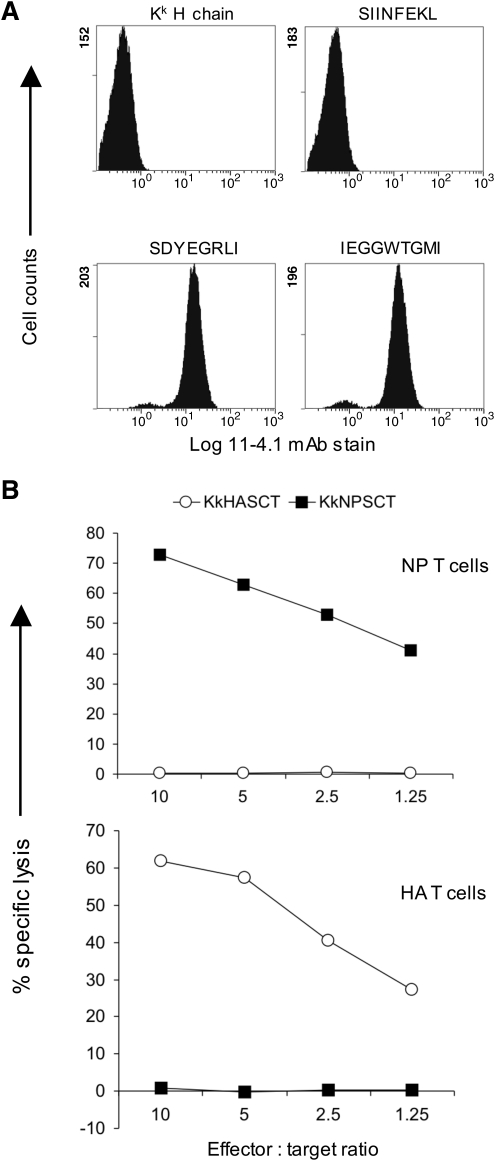
The original SCT format (with wild-type class I H chains) has been reported to work for mouse H-2Kb (6, 16–18, 24, 28, 43, 47, 60, 65), H-2Kk (59, and this article), H-2Db (5, 45), H-2Dd (this article), H-2Ld (55), the nonclassical class Ib allele Qa-1b (3, 30), rat RT1.Al (42), human HLA-A*0201 (4, 14, 20, 22, 23, 28, 41, 44, 56, 67), HLA-B*2705 (12, 56), and the nonclassical class I allele HLA-E (9, 29). Because class I SCTs are loaded with covalently attached peptide, they are expressed at the cell surface independently of the peptide transporter associated with antigen processing (TAP), and a convenient initial test for any new class I SCT construct is to express it in TAP-deficient cells and to assay for cell surface expression using a mAb that only recognizes correctly folded molecules. Expression in normal, TAP-sufficient cells is not sufficient to distinguish between assembly using the covalently linked peptide, or assembly using endogenous cellular peptide. An example of such an approach is shown in Figure 2. When an HLA-A2 SCT encoding the HLA-B27-restricted influenza virus epitope SRYWAIRTR was transfected into TAP-deficient Chinese hamster ovary (CHO) cells, no cell surface expression of correctly folded molecules was detected. However, when the same construct but with the peptide altered to the HLA-A2-restricted HIV epitope SLYNTVATL was transfected into TAP-deficient CHO cells, there was good expression of correctly folded molecules, as detected with the mAbs W6/32 or BB7.2 (Fig. 2). Thus, it is not possible to covalently link any peptide sequence of appropriate length as part of an SCT for it to assemble correctly: importantly, SCTs retain the usual peptide-binding specificity of their constituent class I H chain. Another example is shown in Figure 3 for the mouse class I allele H-2Kk. When a Kk SCT encoding the Kb-restricted ovalbumin-derived epitope SIINFEKL was expressed in TAP-deficient CHO cells, there was no detectable cell surface expression of correctly folded molecules, whereas when the peptide sequence was changed to either one of two Kk-restricted influenza virus-derived epitopes, there was good cell surface expression of assembled molecules (Fig. 3A).
FIG. 2.
Cell surface expression of HLA-A2 SCT molecules presenting different peptides in TAP-deficient cells. TAP2-deficient CHO cells were untransfected (UN), transfected with HLA-A2 SCT encoding the epitope SRYWAIRTR (HLA-B27 peptide), or transfected with HLA-A2 SCT encoding the epitope SLYNTVATL (HLA-A2 peptide). Cells were stained with the conformation dependent mAbs W6/32 (anti-HLA class I) or BB7.2 (anti-HLA-A2). Staining profiles of stable polyclonal cell populations are shown. CHO, Chinese hamster ovary; HLA, human leukocyte antigen; SCT, single-chain trimer; TAP, transporter associated with antigen processing. mAb, monoclonal antibody.
FIG. 3.
Cell surface expression and T cell recognition of H-2Kk SCT molecules presenting different peptides in TAP-deficient cells. (A) TAP2-deficient CHO cells were transfected with either the Kk class I heavy chain alone or Kk SCTs encoding the epitopes SIINFEKL (Kb-restricted from ovalbumin), SDYEGRLI (Kk-restricted from influenza virus nucleoprotein), or IEGGWTGMI (Kk-restricted from influenza virus hemagglutinin), as indicated. Cells were stained with the anti-Kk conformation-dependent mAb 11-4.1. Staining profiles of stable polyclonal cell populations are shown. (B) Cells shown in (A) were used as target cells in a standard 5 h chromium release cytotoxicity assay with T cell lines derived from influenza virus infected H-2k mice as effector cells. Target cells expressed SCTs presenting either the peptide IEGGWTGMI (KkHASCT) or SDYEGRLI (KkNPSCT) as indicated, and were incubated with T cells specific for nucleoprotein (NP T cells) or hemagglutinin (HA T cells).
Recognition of class I SCTs by conformation-dependent mAbs is a useful and rapid assay, but the real test of authentic assembly is functional recognition by lymphocytes. Early studies showed that class I SCTs are recognized efficiently by specific T cells generated in response to normal MHC class I molecules and antigen (14, 53), and subsequently there has been no indication that the presence of a linker sequence between the presented peptide and β2m leads to increased cross-reactivity. Examples of specific T cell recognition of H-2Kk and H-2Dd SCT constructs are shown in Figures 3B and 4B. As expected, recognition of class I SCTs can lead to both target cell lysis and/or cytokine production. NK cells also express receptors that recognize MHC class I, and although in mice they bind to different regions of the class I molecule as compared with T cells, the evidence available suggests that mouse NK cells also recognize class I SCTs (3, 24). Where reported, class I SCTs display greatly enhanced stability on the cell surface as compared with natural MHC class I molecules consisting of noncovalently associated components (47, 65); however, this type of experiment has only been reported for H-2Kb SCTs presenting high-affinity immunodominant epitopes, and it would be useful to measure the relative stability of other SCT molecules: an HLA-E SCT only had a half-life of about 4 h at the cell surface (9).
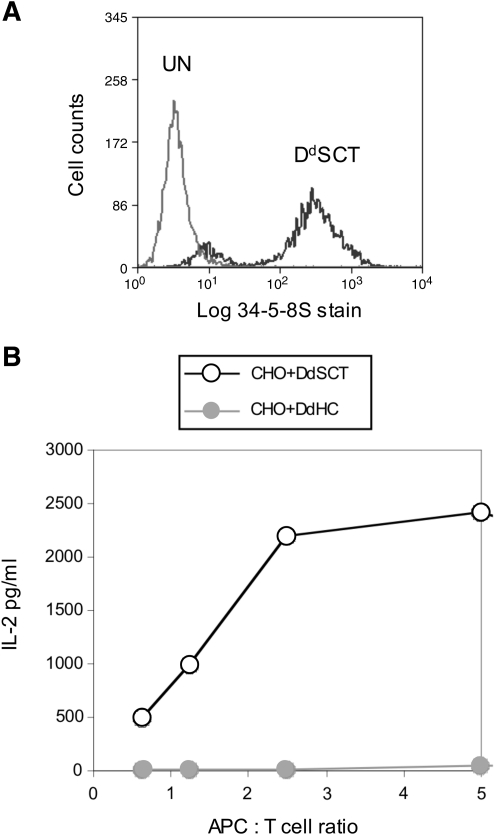
FIG. 4.
Cell surface expression and T cell recognition of a novel H-2Dd SCT construct. (A) CHO cells were either untransfected (UN) or transfected with a Dd SCT encoding the HIV-derived epitope RGPGRAFVTI, as indicated. Cells were stained with the anti-Dd conformation-dependent mAb 34-5-8S. The staining profile of a stable polyclonal cell population is shown. (B) Cells shown in (A) expressing the SCT were used as APCs to stimulate the T cell hybridoma B4.2.3 specific for RGPGRAFVTI presented by Dd, and IL-2 production was measured using standard enzyme-linked immunosorbent assay. CHO cells expressing the Dd class I heavy chain alone were used as a control. A Dd SCT has not been reported previously. APCs, antigen-presenting cells; IL-2, interleukin-2.
Many viruses encode proteins that prevent normal MHC class I assembly and expression at the cell surface as a means of immune evasion. Because of their preassembled nature, SCTs have the potential to overcome some of these evasion strategies. For example, an H-2Kb SCT presenting SIINFEKL was resistant to downregulation by the murine γ-herpesvirus-68 protein MK3 (47), an ER-resident protein that causes the degradation of newly synthesized MHC class I H chains and TAP (2). Similarly, because cell surface expression of SCTs is independent of TAP, viral immune evasion proteins that block TAP peptide transport, such as the herpes simplex virus protein ICP47, should not be able to downregulate SCTs.
Development of the MHC Class I SCT Format
Although the original SCT format works well for a variety of class I H chains presenting immunodominant (high binding affinity) epitopes, one of the attractions of using single-chain MHC class I molecules was to try and increase immune responses against weak, poorly immunogenic epitopes. When this type of low binding affinity peptide epitope was encoded in the SCT format, their assembly was often much less efficient. This must relate to the linker sequence extending from the C-terminal end of the peptide epitope. In natural MHC class I molecules the peptide-binding groove is closed at both ends, and the C-terminal end of the bound peptide interacts with conserved residues on the class I H chain, contributing significantly to peptide-binding affinity. These interactions do not exist in class I SCTs because of the linker, therefore reducing peptide-binding affinity. This may not be important for high-affinity peptides, but may be critical for low-affinity peptides. Efficient immune recognition of class I SCTs presenting immunodominant peptide epitopes indicated that the peptide must be orientated in a manner very similar to the equivalent noncovalently attached peptide. Therefore, it seemed that the linker must protrude out from the C-terminal end of the peptide-binding groove, and this possibility was consistent with a published structure of a decamer peptide bound to HLA-A2 with its C-terminal residue outside the peptide-binding groove (8).
A linker bulging out from one end of the MHC class I peptide-binding groove might be expected to have some detrimental effect on immune recognition. Indeed, the TCR-like antibody 25-D1.16 (46) was found to recognize H-2Kb SCT presenting the ovalbumin-derived peptide SIINFEKL less efficiently than native Kb fully loaded with exogenous SIINFEKL peptide (31). Therefore, Hansen and his colleagues set out to improve the design of SCTs by engineering the class I H chain component to better accommodate this linker. A detailed comparison of the known structures of MHC class I and class II molecules led them to conclude that the tyrosine residue at position 84 (Y84) of the class I H chain plays an important role in closing the peptide C-terminal end of the binding groove (31). Y84 is an invariant residue in classical MHC class I molecules, and makes an important contribution to peptide-binding stability (34). Hansen et al. reasoned that mutation of Y84 to alanine (Y84A) would partially open the class I peptide-binding groove, thereby allowing better accommodation of the linker, and they tested this experimentally using the H-2Kb SCT presenting SIINFEKL (31). As predicted, the Y84A mutation in native Kb class I chains resulted in poor SIINFEKL peptide binding, but in the SCT format this mutation improved both peptide/MHC-specific mAb and T cell recognition (31), indicating better tolerance of the linker. In an ingenious extension to this approach, Hansen and colleagues subsequently devised a method to anchor the presented peptide in the binding groove by the introduction of a new disulfide bond to generate a so-called disulfide trap SCT (dtSCT) (37, 55, 56). They found that when residue 2 of the linker between the peptide and β2m was mutated to a cysteine (L2C) at the same time as Y84 in the class I H chain was changed to cysteine (Y84C), when expressed in cells a new disulfide bond formed between these two cysteine residues, covalently trapping the peptide in the binding groove. Formation of the additional disulfide bond was indicated by more rapid protein migration in nonreducing gels, and functionally because dtSCTs displayed enhanced resistance to peptide displacement by soluble high-affinity competitor peptides (55, 56). Thus, dtSCTs oxidized properly in the ER, transited to the cell surface, and were recognized efficiently by specific T cells.
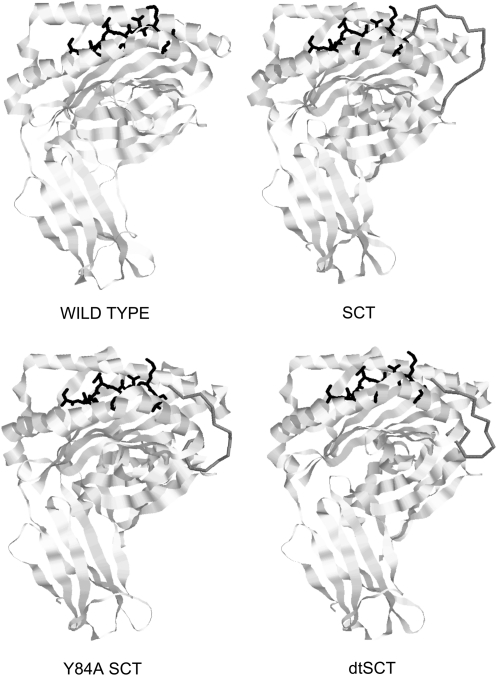
This elegant development of the class I SCT format was put on a firm basis with the later determination of the three-dimensional structures of the three different versions of a H-2Kb SCT presenting the SIINFEKL peptide epitope: original SCT containing unmodified class I H chain, Y84A SCT, and dtSCT (37). Figure 5 illustrates the outline structures of these SCTs compared with wild-type H-2Kb bound to the same peptide, derived from the published coordinates. Although interpretable electron density was observed for the entire peptide-β2m linker (shown as dark gray), no density was observed for the β2m-class I H chain linker (37). The SIINFEKL peptide (shown in black) adopts a very similar conformation in all of the structures: this explains specific T cell recognition of all of the constructs. As predicted, in the original SCT format the linker extending from the C-terminal end of the peptide bulges out from the peptide-binding groove, forming a pronounced loop (Fig. 5). Nevertheless, for the high binding affinity peptide SIINFEKL, this loop does not destabilize peptide binding and still allows immune recognition. As reasoned by Hansen and colleagues, the Y84A mutation in the Kb H chain greatly improved accommodation of the linker within the MHC structure, removing the prominent linker bulge seen with the original SCT (Fig. 5), resulting in a more authentic overall structure. Lastly, crystallography showed directly the formation of the new disulfide bond in a dtSCT, anchoring the presented peptide in place at the same time as allowing improved accommodation of the linker. Functional recognition of the different versions of class I SCTs has been confirmed, but there is no published information comparing TCR binding affinity for the different constructs, for example, using surface plasmon resonance (Biacore). It would be interesting to measure directly TCR binding affinity for an SCT and the equivalent dtSCT, to quantify how much difference improving accommodation of the linker makes to TCR binding. This may vary depending on the specific TCR used.
FIG. 5.
Outline three-dimensional structures of three different versions of an H-2Kb SCT presenting the peptide SIINFEKL, compared with the natural structure. The structures shown were generated using the program RasWin and published coordinates in the Protein Data Bank files 1VAC (wild type), 2QRI (SCT), 2QRS (Y84A SCT), and 2QRT (dtSCT) downloaded from www.pdb.org/. The ovalbumin-derived peptide SIINFEKL is shown in black, the backbone of the linker between SIINFEKL and β2m is shown in dark gray, and the backbone structures of the Kb heavy chain and β2m are shown as light gray ribbons. dtSCT, disulfide trap SCT.
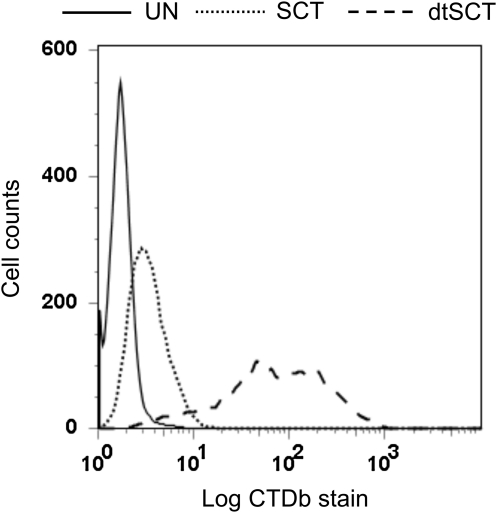
An example of the potential advantage of the dtSCT format is illustrated in Figure 6. In H-2b mice there is a CD8+ T cell response to the male-specific minor histocompatibility antigen H-Y, and two Db-restricted epitopes have been identified, amino acid sequences WMHHNMDLI and KCSRNRQYL. The epitope WMHHNMDLI is immunodominant, whereas the second epitope is more weakly immunogenic (36). When a conventional class I SCT encoding the KCSRNRQYL was generated (using our standard linker sequences, as described above) and expressed in TAP-deficient cells, there was very low cell surface expression of folded molecules (Fig. 6). However, the disulfide trap version of the same SCT gave very good cell surface expression, indicating that the normally low-affinity peptide had been stably anchored in the peptide-binding groove. The immunogenicity of this new construct is currently being tested.
FIG. 6.
Cell surface expression of different versions of an H-2Db SCT presenting a weakly immunogenic epitope in TAP-deficient cells. TAP2-deficient CHO cells were untransfected (UN), transfected with a conventional Db SCT encoding the H-Y epitope KCSRNRQYL (SCT), or transfected with a disulfide trap version of the same SCT (dtSCT), as indicated. Cells were stained with the anti-Db conformation-dependent mAb CTDb. Staining profiles of stable polyclonal cell populations are shown.
Further engineering of MHC class I SCTs may be envisaged: for example, mutations in the class I H chain component that affect CD8 binding may be used to modulate SCT function. Mutations that either increase CD8 binding affinity (62, 64) or decrease CD8 binding affinity (63) have been reported, and these specific mutations may be incorporated into SCTs for various purposes. Increasing CD8 binding affinity to a certain degree enhances the generation of specific CD8+ T cell responses (28, 64), but the binding affinity must not be increased too much or the specificity of the T cell response may be lost (62). Conversely, abolishing CD8 binding may be useful to generate reagents that will select for T cells with high-affinity TCRs, not dependent on the CD8 coreceptor for function (63).
Biochemical Properties of MHC Class I SCTs
Although many different MHC class I SCTs have been reported, little biochemical information is available for most of them. The most detailed biochemical studies have used H-2Kb SCTs encoding high-affinity peptide epitopes, such as SIINFEKL. Initial biochemical experiments used immunoprecipitation and western blotting to demonstrate that SCT constructs remain covalently intact within cells, and their covalently attached peptide is not cleaved before simply re-binding (65). SCTs may be detected on western blots by using epitope tags introduced within the class I H chain or at the C-terminal cytoplasmic end, or by using anti-β2m antibodies. By using mAbs that recognize either only open class I H chains or only correctly folded Kb molecules, Hansen and colleagues showed that at the steady state the SCT, unlike Kb H chain alone, has a very low proportion of unfolded molecules in cells, and also migrates at the higher, expected molecular weight during sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (65). To show that the SIINFEKL peptide was not being cleaved from the SCT and re-binding as free peptide, immunoprecipitation was performed using the TCR-like mAb 25-D1.16. SCT molecules precipitated from cells migrated at a slightly higher molecular weight during SDS-PAGE than β2m-Kb fusion molecules loaded with exogenous peptide, demonstrating that the SCT molecules retain covalently (linker) attached peptide (31). The low level of unassembled SCT molecules within cells suggested that class I SCTs assemble and leave the ER very rapidly, and this is supported by the one pulse chase experiment that has been reported (47). Whether other class I SCTs fold as rapidly as the Kb SCT, or whether the rate of folding and trafficking is still dependent on the class I H chain component remains to be determined. It would be interesting to know whether a class I H chain that normally folds slowly in the ER (e.g., HLA-B27) folds rapidly when expressed in the SCT format, and whether the introduction of a new disulfide bond as in dtSCTs affects the rate of folding. Similarly, there is little published experimental data concerning the ER chaperone dependence of class I SCT folding. It is clear that class I SCTs are expressed efficiently at the cell surface in TAP-deficient (e.g., see Figs. 2 and 3) and β2m-deficient cells (45), and therefore are predicted to be independent of tapasin and the peptide loading complex. In agreement with this, a Kb SCT displayed no detectable steady-state association with tapasin or TAP (47). Studies investigating the possible roles of other accessory proteins implicated in normal MHC class I assembly, that is, calnexin, calreticulin, and protein disulfide isomerases, have not been reported for class I SCT folding. One study showed that an HLA-B27 SCT was more resistant to reduction by dithiothreitol than the equivalent β2m-H chain fusion (12), indicating efficient folding.
With the development and improvement of the class I SCT format, as described above, biochemical characterization of Y84A and dtSCTs has also been reported. In the H-2Kb SIINFEKL SCT system, introduction of the Y84A mutation improved recognition both by the mAb 25-D1.16 and by specific T cells (31), consistent with the structural data showing improved accommodation of the linker (Fig. 5). The Y84A mutation did not affect the SCT stability at the cell surface, but did improve exclusion of competitor peptides from the binding groove of the SCT approximately fivefold (31). Interestingly, both these forms of SCT did not exclude competitor peptide anywhere near as efficiently as native Kb molecules loaded with SIINFEKL peptide, even though they were much more stable at the cell surface than native Kb molecules (31). Therefore, it seems that the remarkable stability of these class I SCTs is due to their ability to efficiently rebind the covalently attached peptide when it does become dissociated, rather than an ability to prevent peptide dissociation.
dtSCTs have been reported for the H-2Kb (37, 55), H-2Ld (55), and HLA-A2 (56) MHC class I alleles. As shown in Figure 6, we have also generated an H-2Db dtSCT. For H-2Kb the formation of the new disulfide bond has been verified by structural studies, and for the other alleles it may be inferred from more rapid migration on nonreducing SDS-PAGE (55). Although introducing the requirement for formation of an additional intramolecular disulfide bond in the ER, which could theoretically slow down protein folding and increase ER retention, the evidence available actually suggests more rapid assembly and egress from the ER, at least for Ld SCTs (55). H-2Ld is an unusual class I allele because of its relatively weak association with peptide and β2m (1), and it would be interesting to test the rate of assembly of dtSCTs containing other class I H chains, preferably directly in pulse chase experiments. The outstanding characteristic of dtSCTs is their enhanced ability to exclude competitor peptides; a disulfide trap made the Kb SIINFEKL SCT at least 100-fold more refractory than the other forms of SCT to exogenous peptide binding (37). This is also reflected in their increased thermostability (37). Most importantly, for the first time dtSCTs should allow the generation of stable MHC class I molecules presenting low-affinity peptide epitopes, thereby offering more possibilities for vaccine development.
Applications of MHC Class I SCTs
There are three main areas where the unique properties of MHC class I SCTs have been exploited: generation of CD8+ T cell responses, generation of reagents for the detection of specific CD8+ T cell responses, and as powerful tools for addressing fundamental questions in immunology. In addition, SCTs have been used for re-targeting CD8+ T cell responses, and as reagents for generating antibodies with TCR-like specificity. This diversity of applications emphasizes the utility of the MHC class I SCT format.
Because of their unusual properties, MHC class I SCTs have important potential advantages for generating CD8+ T cell responses, both in vivo and in vitro. Their greatly increased cell surface stability is predicted to increase their ability to stimulate T cells (49, 66), and indeed when cells with identical levels of SCT or peptide-loaded native class I were compared for their ability to stimulate a T cell hybridoma in vitro, the class I SCT gave significantly greater responses (6). An H-2Db class I SCT also showed enhanced immunogenicity for generating CD8+ T cell responses in vivo, even when compared with a preprocessed epitope mini-gene targeted to the ER (45). An attractive option is to use class I SCTs in the form of DNA vaccines (15). DNA vaccines are relatively safe, cheap, and easy to administer, and they also avoid problems with antivector immune responses. Various studies have used class I SCTs as DNA vaccines, and found them to be effective (4, 16, 17, 20, 22, 23, 28, 43, 67). Obviously, it is essential to know the amino acid sequence of an important CD8+ epitope before a class I SCT can be used as a DNA vaccine, and if a pathogen can evade immune recognition by sequence variation of this epitope, it is less likely to make an effective vaccine. For this reason, class I SCTs may be most useful in vaccines designed to combat cancer. It may also be possible to use a vaccine containing a combination of different SCTs presenting different epitopes. Another concern about the use of class I SCTs as DNA vaccines is provision of the CD4+ T cell help required for optimal CD8+ T cell responses. However, inclusion of a universal helper epitope within the same DNA vaccine as the class I SCT is able to enhance CD8+ T cell responses, and improve immune protection (23, 28). It is also possible to enhance responses to class I SCT DNA vaccines by mutating the class I H chain component to enhance CD8 binding (28), or by prolonging the survival of DNA transduced dendritic cells by coexpression of antiapoptotic proteins in the DNA vaccine (16). As an alternative to vaccination, adoptive T cell therapy is a promising option for the treatment of some cancers, in which high numbers of antigen-specific T cells are expanded in vitro before transfer into patients. Artificial antigen-presenting cells expressing class I SCTs on their surface are an efficient tool for the expansion of human T cells (41).
Multimers of soluble MHC class I presenting defined peptides have become essential tools for quantifying specific CD8+ T cell responses in patients and in vaccine trials, as well as being important research reagents (63). Generally, recombinant MHC class I H chain lacking the transmembrane region and cytoplasmic tail is expressed in bacteria together with β2m, denatured, and then refolded in vitro in the presence of synthetic peptide epitope. A biotinylation site is included at the C-terminal end of the class I H chain to allow multimer formation via streptavidin, conjugated to a fluorescent dye for observation. Such reagents frequently work well, but certain class I allele/epitope combinations can have problems related to peptide dissociation, and there is anecdotal evidence that it is not possible to generate multimeric staining reagents using traditional methods for some peptide epitopes. The class I SCT format has the ability to stabilize peptides with low binding affinities, thereby allowing the generation of a greater diversity of T cell staining reagents. Conventional class I SCTs have been shown to stain T cells in an MHC/peptide specific manner (14, 31, 37, 42), but the best strategy for preventing peptide dissociation in T cell staining reagents comes from development of the dtSCT format by Hansen and colleagues (37). Using a disulfide bond to covalently trap peptide in the binding groove of class I molecules should in principle allow the generation of staining reagents for any CD8+ T cell specificity. The only complication may come with peptide epitopes that themselves contain a cysteine residue.
In addition to the more applied applications outlined above, MHC class I SCTs have also been used to address important fundamental questions in immunology. Because they fold up independently of the usual class I assembly pathway, it is possible to alter the dimensions of a class I SCT by inserting additional immunoglobulin-like domains between the class I transmembrane region and α3 domain, and the molecule will still fold up correctly for TCR and NK receptor recognition. This allowed a rigorous test of the role of ligand dimensions in triggering receptors on CD8+ T cells (5, 6), and on NK cells (3). These studies have provided strong evidence that size-based segregation of molecules at cell–cell contact interfaces is important in delivering signals to lymphocytes. Transgenic mice expressing an MHC class I SCT as their only MHC class I molecule have been used in elegant studies investigating NK cell licensing and education (24), and development of the CD8+ T cell repertoire (60). An SCT transgenic mouse was used to demonstrate that NK cells are licensed by inhibitory receptors that engage self MHC class I molecules during development (24). SCT transgenic mice provided direct in vivo evidence that both the MHC and peptide specificity of the CD8+ T cell repertoire is imparted by positive selection in the thymus (60). Hudrisier et al. used an SCT approach to investigate the significance of trogocytosis (18), the process in which lymphocytes actively capture small fragments of plasma membrane from cells with which they form an immune synapse. In vitro, different states of CD8+ T cell activation were found to correlate with different levels of peptide-MHC trogocytosis (18). The preassembled nature of a class I SCT makes it an ideal tool for investigating peptide-MHC transfer between cells.
There is currently a lot of interest in being able to re-target pre-existing antiviral CD8+ T cell immunity against tumors in patients. Most individuals have memory CD8+ T cells against common viruses such as influenza virus, and these could easily be boosted to expand effector cell populations within the individual concerned. If tumor cells could be made targets for these virus-specific effector lymphocytes, the tumor may be eliminated or at least controlled. Antibody mediated targeting of soluble MHC class I SCT molecules may be the best method for adopting this strategy (40, 44), but is dependent on the availability of suitable antibody specificities. Here again, class I SCTs may be able to help. Class I SCTs have been reported as effective reagents for generating mAbs with TCR-like specificities, only recognizing MHC class I molecules when they are loaded with a particular peptide (47, 65). Such peptide-MHC-specific mAbs have generally been isolated from antibody libraries using phage display, but in principle immunization with class I SCTs should also be able to generate such mAbs. Antibodies with specificities for tumor epitopes complexed with MHC class I have been reported (35), and coupling such specificities to class I SCTs presenting viral epitopes is a promising approach for immunotherapy of cancer.
Future Prospects
Although MHC class I SCTs have now been used in a significant number of publications, their full potential has undoubtedly not yet been realized, and their true value has perhaps not yet been widely appreciated. The flexibility of the SCT format should allow an increasing diversity of future applications. In addition to their more translational uses in vaccine studies, immunotherapy, and quantifying specific T cell responses, MHC class I SCTs will allow important questions in CD8+ T cell and NK cell biology to be addressed: how do self-peptide MHC class I complexes contribute to T cell activation and deliver homeostatic signals to mature T cells? How do peptide epitope agonists compare with peptide antagonists in vivo? What is the peptide specificity of MHC class I binding receptors on NK cells, both mouse and human? How do MHC class I molecules influence development of the nervous system? These and many other questions are amenable to investigation using MHC class I SCTs presenting single, defined peptides.
Abbreviations Used
- APCs
antigen-presenting cells
- β2m
β2-microglobulin
- CHO
Chinese hamster ovary
- dtSCT
disulfide trap single-chain trimer
- ER
endoplasmic reticulum
- H chain
heavy chain
- HLA
human leukocyte antigen
- IL-2
interleukin-2
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- NK
natural killer
- SCT
single-chain trimer
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- TAP
transporter associated with antigen processing
- TCR
T cell receptor
Acknowledgments
We thank Tom Serwold and Nilabh Shastri (University of California) for TAP2-deficient CHO cells, David Margulies (National Institutes of Health, Bethesda) for the B4.2.3 T cell hybridoma, and Leukaemia & Lymphoma Research (London), the Wellcome Trust, and the Medical Research Council (United Kingdom) for funding work in our laboratory.
References
- 1.Balendiran GK. Solheim JC. Young AC. Hansen TH. Nathenson SG. Sacchettini JC. The three-dimensional structure of an H-2Ld-peptide complex explains the unique interaction of Ld with beta-2 microglobulin and peptide. Proc Natl Acad Sci U S A. 1997;94:6880–6885. doi: 10.1073/pnas.94.13.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boname JM. de Lima BD. Lehner PJ. Stevenson PG. Viral degradation of the MHC class I peptide loading complex. Immunity. 2004;20:305–317. doi: 10.1016/s1074-7613(04)00047-0. [DOI] [PubMed] [Google Scholar]
- 3.Brzostek J. Chai JG. Gebhardt F. Busch DH. Zhao R. van der Merwe PA. Gould KG. Ligand dimensions are important in controlling NK-cell responses. Eur J Immunol. 2010;40:2050–2059. doi: 10.1002/eji.201040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung YK. Cheng SC. Sin FW. Chan KT. Xie Y. Induction of T-cell response by a DNA vaccine encoding a novel HLA-A*0201 severe acute respiratory syndrome coronavirus epitope. Vaccine. 2007;25:6070–6077. doi: 10.1016/j.vaccine.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhuri K. Parker M. Milicic A. Cole DK. Shaw MK. Sewell AK. Stewart-Jones G. Dong T. Gould KG. van der Merwe PA. Peptide-major histocompatibility complex dimensions control proximal kinase-phosphatase balance during T cell activation. J Biol Chem. 2009;284:26096–26105. doi: 10.1074/jbc.M109.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhuri K. Wiseman D. Brown MH. Gould K. van der Merwe PA. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 7.Chung DH. Dorfman J. Plaksin D. Natarajan K. Belyakov IM. Hunziker R. Berzofsky JA. Yokoyama WM. Mage MG. Margulies DH. NK and CTL recognition of a single chain H-2Dd molecule: distinct sites of H-2Dd interact with NK and TCR. J Immunol. 1999;163:3699–3708. [PubMed] [Google Scholar]
- 8.Collins EJ. Garboczi DN. Wiley DC. Three-dimensional structure of a peptide extending from one end of a class I MHC binding site. Nature. 1994;371:626–629. doi: 10.1038/371626a0. [DOI] [PubMed] [Google Scholar]
- 9.Crew MD. Cannon MJ. Phanavanh B. Garcia-Borges CN. An HLA-E single chain trimer inhibits human NK cell reactivity towards porcine cells. Mol Immunol. 2005;42:1205–1214. doi: 10.1016/j.molimm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Datwani A. McConnell MJ. Kanold PO. Micheva KD. Busse B. Shamloo M. Smith SJ. Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dela Cruz CS. Tan R. Rowland-Jones SL. Barber BH. Creating HIV-1 reverse transcriptase cytotoxic T lymphocyte target structures by HLA-A2 heavy chain modifications. Int Immunol. 2000;12:1293–1302. doi: 10.1093/intimm/12.9.1293. [DOI] [PubMed] [Google Scholar]
- 12.Fussell H. Nesbeth D. Lenart I. Campbell EC. Lynch S. Santos S. Gould K. Powis SJ. Antoniou AN. Novel detection of in vivo HLA-B27 conformations correlates with ankylosing spondylitis association. Arthritis Rheum. 2008;58:3419–3424. doi: 10.1002/art.23990. [DOI] [PubMed] [Google Scholar]
- 13.Godeau F. Luescher IF. Ojcius DM. Saucier C. Mottez E. Cabanie L. Kourilsky P. Purification and ligand binding of a soluble class I major histocompatibility complex molecule consisting of the first three domains of H-2Kd fused to beta 2-microglobulin expressed in the baculovirus-insect cell system. J Biol Chem. 1992;267:24223–24229. [PubMed] [Google Scholar]
- 14.Greten TF. Korangy F. Neumann G. Wedemeyer H. Schlote K. Heller A. Scheffer S. Pardoll DM. Garbe AI. Schneck JP. Manns MP. Peptide-beta2-microglobulin-MHC fusion molecules bind antigen-specific T cells and can be used for multivalent MHC-Ig complexes. J Immunol Methods. 2002;271:125–135. doi: 10.1016/s0022-1759(02)00346-0. [DOI] [PubMed] [Google Scholar]
- 15.Hansen TH. Lybarger L. Exciting applications of single chain trimers of MHC-I molecules. Cancer Immunol Immunother. 2006;55:235–236. doi: 10.1007/s00262-005-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang B. Mao CP. Peng S. He L. Hung CF. Wu TC. Intradermal administration of DNA vaccines combining a strategy to bypass antigen processing with a strategy to prolong dendritic cell survival enhances DNA vaccine potency. Vaccine. 2007;25:7824–7831. doi: 10.1016/j.vaccine.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang CH. Peng S. He L. Tsai YC. Boyd DA. Hansen TH. Wu TC. Hung CF. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther. 2005;12:1180–1186. doi: 10.1038/sj.gt.3302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudrisier D. Riond J. Garidou L. Duthoit C. Joly E. T cell activation correlates with an increased proportion of antigen among the materials acquired from target cells. Eur J Immunol. 2005;35:2284–2294. doi: 10.1002/eji.200526266. [DOI] [PubMed] [Google Scholar]
- 19.Huh GS. Boulanger LM. Du H. Riquelme PA. Brotz TM. Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung CF. Calizo R. Tsai YC. He L. Wu TC. A DNA vaccine encoding a single-chain trimer of HLA-A2 linked to human mesothelin peptide generates anti-tumor effects against human mesothelin-expressing tumors. Vaccine. 2007;25:127–135. doi: 10.1016/j.vaccine.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 21.Ignatowicz L. Winslow G. Bill J. Kappler J. Marrack P. Cell surface expression of class II MHC proteins bound by a single peptide. J Immunol. 1995;154:3852–3862. [PubMed] [Google Scholar]
- 22.Jaramillo A. Narayanan K. Campbell LG. Benshoff ND. Lybarger L. Hansen TH. Fleming TP. Dietz JR. Mohanakumar T. Recognition of HLA-A2-restricted mammaglobin-A-derived epitopes by CD8+ cytotoxic T lymphocytes from breast cancer patients. Breast Cancer Res Treat. 2004;88:29–41. doi: 10.1007/s10549-004-8918-1. [DOI] [PubMed] [Google Scholar]
- 23.Kim S. Li L. McMurtrey CP. Hildebrand WH. Weidanz JA. Gillanders WE. Diamond MS. Hansen TH. Single-chain HLA-A2 MHC trimers that incorporate an immundominant peptide elicit protective T cell immunity against lethal West Nile virus infection. J Immunol. 2010;184:4423–4430. doi: 10.4049/jimmunol.0903955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S. Poursine-Laurent J. Truscott SM. Lybarger L. Song YJ. Yang L. French AR. Sunwoo JB. Lemieux S. Hansen TH. Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 25.Kurts C. Robinson BW. Knolle PA. Cross-priming in health and disease. Nat Rev Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 26.Lee L. Loftus D. Appella E. Margulies DH. Mage M. A recombinant single-chain HLA-A2.1 molecule, with a cis active beta-2-microglobulin domain, is biologically active in peptide binding and antigen presentation. Hum Immunol. 1996;49:28–37. doi: 10.1016/0198-8859(96)00056-0. [DOI] [PubMed] [Google Scholar]
- 27.Lee L. McHugh L. Ribaudo RK. Kozlowski S. Margulies DH. Mage MG. Functional cell surface expression by a recombinant single-chain class I major histocompatibility complex molecule with a cis-active beta 2-microglobulin domain. Eur J Immunol. 1994;24:2633–2639. doi: 10.1002/eji.1830241110. [DOI] [PubMed] [Google Scholar]
- 28.Li L. Herndon JM. Truscott SM. Hansen TH. Fleming TP. Goedegebuure P. Gillanders WE. Engineering superior DNA vaccines: MHC class I single chain trimers bypass antigen processing and enhance the immune response to low affinity antigens. Vaccine. 2010;28:1911–1918. doi: 10.1016/j.vaccine.2009.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lilienfeld BG. Crew MD. Forte P. Baumann BC. Seebach JD. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation. 2007;14:126–134. doi: 10.1111/j.1399-3089.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 30.Lu L. Ikizawa K. Hu D. Werneck MB. Wucherpfennig KW. Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lybarger L. Yu YY. Miley MJ. Fremont DH. Myers N. Primeau T. Truscott SM. Connolly JM. Hansen TH. Enhanced immune presentation of a single-chain major histocompatibility complex class I molecule engineered to optimize linkage of a C-terminally extended peptide. J Biol Chem. 2003;278:27105–27111. doi: 10.1074/jbc.M303716200. [DOI] [PubMed] [Google Scholar]
- 32.Madden DR. The three-dimensional structure of peptide-MHC complexes. Annu Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 33.Mage MG. Lee L. Ribaudo RK. Corr M. Kozlowski S. McHugh L. Margulies DH. A recombinant, soluble, single-chain class I major histocompatibility complex molecule with biological activity. Proc Natl Acad Sci U S A. 1992;89:10658–10662. doi: 10.1073/pnas.89.22.10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumura M. Fremont DH. Peterson PA. Wilson IA. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992;257:927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- 35.Michaeli Y. Denkberg G. Sinik K. Lantzy L. Chih-Sheng C. Beauverd C. Ziv T. Romero P. Reiter Y. Expression hierarchy of T cell epitopes from melanoma differentiation antigens: unexpected high level presentation of tyrosinase-HLA-A2 Complexes revealed by peptide-specific, MHC-restricted, TCR-like antibodies. J Immunol. 2009;182:6328–6341. doi: 10.4049/jimmunol.0801898. [DOI] [PubMed] [Google Scholar]
- 36.Millrain M. Chandler P. Dazzi F. Scott D. Simpson E. Dyson PJ. Examination of HY response: T cell expansion, immunodominance, and cross-priming revealed by HY tetramer analysis. J Immunol. 2001;167:3756–3764. doi: 10.4049/jimmunol.167.7.3756. [DOI] [PubMed] [Google Scholar]
- 37.Mitaksov V. Truscott SM. Lybarger L. Connolly JM. Hansen TH. Fremont DH. Structural engineering of pMHC reagents for T cell vaccines and diagnostics. Chem Biol. 2007;14:909–922. doi: 10.1016/j.chembiol.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mottez E. Jaulin C. Godeau F. Choppin J. Levy JP. Kourilsky P. A single-chain murine class I major transplantation antigen. Eur J Immunol. 1991;21:467–471. doi: 10.1002/eji.1830210232. [DOI] [PubMed] [Google Scholar]
- 39.Mottez E. Langlade-Demoyen P. Gournier H. Martinon F. Maryanski J. Kourilsky P. Abastado JP. Cells expressing a major histocompatibility complex class I molecule with a single covalently bound peptide are highly immunogenic. J Exp Med. 1995;181:493–502. doi: 10.1084/jem.181.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novak H. Noy R. Oved K. Segal D. Wels WS. Reiter Y. Selective antibody-mediated targeting of class I MHC to EGFR-expressing tumor cells induces potent antitumor CTL activity in vitro and in vivo. Int J Cancer. 2007;120:329–336. doi: 10.1002/ijc.22168. [DOI] [PubMed] [Google Scholar]
- 41.Obermann S. Petrykowska S. Manns MP. Korangy F. Greten TF. Peptide-beta2-microglobulin-major histocompatibility complex expressing cells are potent antigen-presenting cells that can generate specific T cells. Immunology. 2007;122:90–97. doi: 10.1111/j.1365-2567.2007.02616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohashi T. Nagai M. Okada H. Takayanagi R. Shida H. Activation and detection of HTLV-I Tax-specific CTLs by epitope expressing single-chain trimers of MHC Class I in a rat model. Retrovirology. 2008;5:90. doi: 10.1186/1742-4690-5-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ordaz ML. Larmonier N. Lybarger L. DC-expressed MHC class I single-chain trimer-based vaccines prime cytotoxic T lymphocytes against exogenous but not endogenous antigens. Cell Immunol. 2010;262:141–149. doi: 10.1016/j.cellimm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Oved K. Lev A. Noy R. Segal D. Reiter Y. Antibody-mediated targeting of human single-chain class I MHC with covalently linked peptides induces efficient killing of tumor cells by tumor or viral-specific cytotoxic T lymphocytes. Cancer Immunol Immunother. 2005;54:867–879. doi: 10.1007/s00262-005-0666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmowski MJ. Parker M. Choudhuri K. Chiu C. Callan MF. van der Merwe PA. Cerundolo V. Gould KG. A single-chain H-2Db molecule presenting an influenza virus nucleoprotein epitope shows enhanced ability at stimulating CD8+ T cell responses in vivo. J Immunol. 2009;182:4565–4571. doi: 10.4049/jimmunol.0803893. [DOI] [PubMed] [Google Scholar]
- 46.Porgador A. Yewdell JW. Deng Y. Bennink JR. Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 47.Primeau T. Myers NB. Yu YY. Lybarger L. Wang X. Truscott SM. Hansen TH. Connolly JM. Applications of major histocompatibility complex class I molecules expressed as single chains. Immunol Res. 2005;32:109–121. doi: 10.1385/ir:32:1-3:109. [DOI] [PubMed] [Google Scholar]
- 48.Rhode PR. Burkhardt M. Jiao J. Siddiqui AH. Huang GP. Wong HC. Single-chain MHC class II molecules induce T cell activation and apoptosis. J Immunol. 1996;157:4885–4891. [PubMed] [Google Scholar]
- 49.Rudd BD. Brien JD. Davenport MP. Nikolich-Zugich J. Cutting edge: TLR ligands increase TCR triggering by slowing peptide-MHC class I decay rates. J Immunol. 2008;181:5199–5203. doi: 10.4049/jimmunol.181.8.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sylvester-Hvid C. Nielsen LL. Hansen NJ. Pedersen LO. Buus S. A single-chain fusion molecule consisting of peptide, major histocompatibility gene complex class I heavy chain and beta2-microglobulin can fold partially correctly, but binds peptide inefficiently. Scand J Immunol. 1999;50:355–362. doi: 10.1046/j.1365-3083.1999.00601.x. [DOI] [PubMed] [Google Scholar]
- 51.Tafuro S. Meier UC. Dunbar PR. Jones EY. Layton GT. Hunter MG. Bell JI. McMichael AJ. Reconstitution of antigen presentation in HLA class I-negative cancer cells with peptide-beta2m fusion molecules. Eur J Immunol. 2001;31:440–449. doi: 10.1002/1521-4141(200102)31:2<440::aid-immu440>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 52.Toshitani K. Braud V. Browning MJ. Murray N. McMichael AJ. Bodmer WF. Expression of a single-chain HLA class I molecule in a human cell line: presentation of exogenous peptide and processed antigen to cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1996;93:236–240. doi: 10.1073/pnas.93.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tourdot S. Gould KG. Competition between MHC class I alleles for cell surface expression alters CTL responses to influenza A virus. J Immunol. 2002;169:5615–5621. doi: 10.4049/jimmunol.169.10.5615. [DOI] [PubMed] [Google Scholar]
- 54.Tourdot S. Nejmeddine M. Powis SJ. Gould KG. Different MHC class I heavy chains compete with each other for folding independently of beta 2-microglobulin and peptide. J Immunol. 2005;174:925–933. doi: 10.4049/jimmunol.174.2.925. [DOI] [PubMed] [Google Scholar]
- 55.Truscott SM. Lybarger L. Martinko JM. Mitaksov VE. Kranz DM. Connolly JM. Fremont DH. Hansen TH. Disulfide bond engineering to trap peptides in the MHC class I binding groove. J Immunol. 2007;178:6280–6289. doi: 10.4049/jimmunol.178.10.6280. [DOI] [PubMed] [Google Scholar]
- 56.Truscott SM. Wang X. Lybarger L. Biddison WE. McBerry C. Martinko JM. Connolly JM. Linette GP. Fremont DH. Hansen TH. Carreno BM. Human major histocompatibility complex (MHC) class I molecules with disulfide traps secure disease-related antigenic peptides and exclude competitor peptides. J Biol Chem. 2008;283:7480–7490. doi: 10.1074/jbc.M709935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uger RA. Barber BH. Creating CTL targets with epitope-linked beta 2-microglobulin constructs. J Immunol. 1998;160:1598–1605. [PubMed] [Google Scholar]
- 58.Uger RA. Chan SM. Barber BH. Covalent linkage to beta2-microglobulin enhances the MHC stability and antigenicity of suboptimal CTL epitopes. J Immunol. 1999;162:6024–6028. [PubMed] [Google Scholar]
- 59.Vest Hansen N. Ostergaard Pedersen L. Stryhn A. Buus S. Phage display of peptide/major histocompatibility class I complexes. Eur J Immunol. 2001;31:32–38. doi: 10.1002/1521-4141(200101)31:1<32::aid-immu32>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 60.Wang B. Primeau TM. Myers N. Rohrs HW. Gross ML. Lybarger L. Hansen TH. Connolly JM. A single peptide-MHC complex positively selects a diverse and specific CD8 T cell repertoire. Science. 2009;326:871–874. doi: 10.1126/science.1177627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White J. Crawford F. Fremont D. Marrack P. Kappler J. Soluble class I MHC with beta2-microglobulin covalently linked peptides: specific binding to a T cell hybridoma. J Immunol. 1999;162:2671–2676. [PubMed] [Google Scholar]
- 62.Wooldridge L. Clement M. Lissina A. Edwards ES. Ladell K. Ekeruche J. Hewitt RE. Laugel B. Gostick E. Cole DK. Debets R. Berrevoets C. Miles JJ. Burrows SR. Price DA. Sewell AK. MHC class I molecules with Superenhanced CD8 binding properties bypass the requirement for cognate TCR recognition and nonspecifically activate CTLs. J Immunol. 2010;184:3357–3366. doi: 10.4049/jimmunol.0902398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wooldridge L. Lissina A. Cole DK. van den Berg HA. Price DA. Sewell AK. Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology. 2009;126:147–164. doi: 10.1111/j.1365-2567.2008.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wooldridge L. Lissina A. Vernazza J. Gostick E. Laugel B. Hutchinson SL. Mirza F. Dunbar PR. Boulter JM. Glick M. Cerundolo V. van den Berg HA. Price DA. Sewell AK. Enhanced immunogenicity of CTL antigens through mutation of the CD8 binding MHC class I invariant region. Eur J Immunol. 2007;37:1323–1333. doi: 10.1002/eji.200636765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu YY. Netuschil N. Lybarger L. Connolly JM. Hansen TH. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J Immunol. 2002;168:3145–3149. doi: 10.4049/jimmunol.168.7.3145. [DOI] [PubMed] [Google Scholar]
- 66.Zehn D. Cohen CJ. Reiter Y. Walden P. Extended presentation of specific MHC-peptide complexes by mature dendritic cells compared to other types of antigen-presenting cells. Eur J Immunol. 2004;34:1551–1560. doi: 10.1002/eji.200324355. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y. Li S. Shan M. Pan X. Zhuang K. He L. Gould K. Tien P. Hepatitis B virus core antigen epitopes presented by HLA-A2 single-chain trimers induce functional epitope-specific CD8+ T-cell responses in HLA-A2.1/Kb transgenic mice. Immunology. 2007;121:105–112. doi: 10.1111/j.1365-2567.2007.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao H. Stet RJ. Skjodt K. Savelkoul HF. Expression and characterization of recombinant single-chain salmon class I MHC fused with beta2-microglobulin with biological activity. Fish Shellfish Immunol. 2008;24:459–466. doi: 10.1016/j.fsi.2008.01.003. [DOI] [PubMed] [Google Scholar]