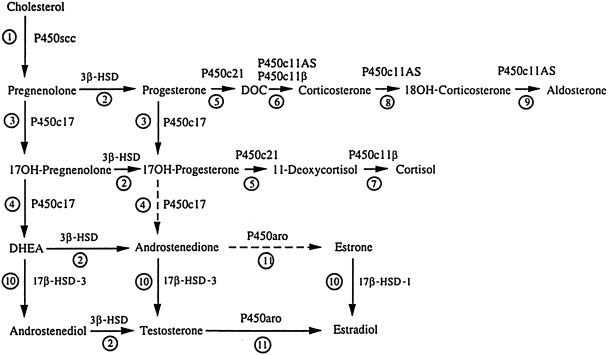
FIG. 1.
Integrated view of human steroidogenesis, showing adrenal and gonadal pathways. Reaction 1: P450scc converts cholesterol to pregnenolone. Reaction 2: 3β-hydroxysteroid dehydrogenase (3β-HSD) converts Δ5 steroids [pregnenolone, 17OH-pregnenolone, dehydroepiandrosterone (DHEA), androstenediol] to the corresponding Δ4 steroids (progesterone, androstenedione, testosterone). Reaction 3: P450c17 catalyzes the 17α-hydroxylation of pregnenolone and progesterone. Reaction 4: The 17,20-lyase activity of P450c17 converts 17OH-pregnenolone to DHEA; the conversion of 17OH-progesterone to androstenedione occurs in cattle and rodents, but human P450c17 cannot catalyze this reaction efficiently. Reaction 5: P450c21 catalyzes the 21-hydroxylation of progesterone and 17OH-progesterone. Reaction 6: Deoxycorticosterone (DOC) can be converted to corticosterone by either P450c11AS (in the adrenal zona glomerulosa) or P450c11β (in the adrenal zona fasciculata). Reaction 7: P450c11β converts 11-deoxycortisol to cortisol. Reactions 8 and 9: P450c11AS catalyzes 18 hydroxylase (reaction 8) and 18 methyl oxidase activities (reaction 9) to produce aldosterone in the adrenal zona glomerulosa. Reaction 10: Two isozymes of 17βHSD activate sex steroids: 17β-HSD1 produces estradiol and 17β-HSD3 produces androgens. In peripheral tissues 17β-HSD5 has similar activity to 17β-HSD3, and 17β-HSD2 and 4 catalyze the “reverse” reactions to inactivate sex steroids. Reaction 11: P450aro aromatizes C19 androgenic steroids to C18 estrogens.