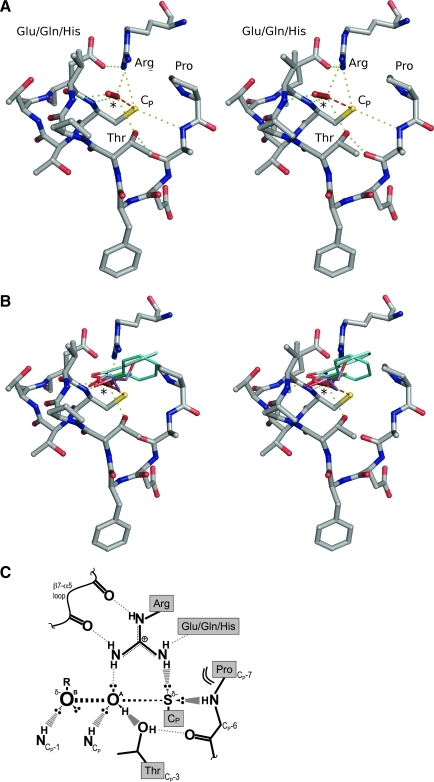
FIG. 3.
The peroxidatic active site. (A) Stereoview of the FF Prx active site with a bound H2O2 molecule. Shown are the highly conserved contiguous CP-loop and the first turn of α2 plus the active-site Arg and an associated Glu/Gln/His supporting residue. The proximity to the CP and the hydrogen-bonding interactions (green dotted lines) highlight the importance of the conserved Pro, Thr, and Arg in binding and activating the peroxide substrate (*) and in activating the CP sulfhydryl for attack of the substrate oxygen atom (orange dashed line). The Glu/Gln/His residue, although not 100% conserved across all Prxs, is important as a hydrogen-bond acceptor positioning the conserved Arg. This figure was created by using ApTpx (entry 37 in Table 1), colored by atom (C, gray; N, blue; O, red; S, yellow). (B) Stereoview of an overlay of the H2O2-bound Prx from (A) with benzoate (cyan tones, entries 38 and 24 in Table 1), acetate (green tones, entries 35 and 56 in Table 1), ethanediol (light blue, entry 29 in Table 1) and glycerol (violet, entry 36 in Table 1), as seen bound in other Prx structures. Protein atoms are shown only for the Prx bound to H2O2, and protein coloring and hydrogen bonds to H2O2 (*) are as in (A). (C) Cartoon representation of the active-site transition-state conformation. The stabilizing interactions between key atoms from the backbone and the four conserved residues, and with the H2O2 substrate, are indicated. In the transition state, a bond is forming between the S atom of the CP and the OA of H2O2, and a bond is breaking between the OA and OB atoms of H2O2. The geometry of the active site is ideal for stabilizing the larger distance between the OA and OB atoms as the bond is broken. (A, B) were prepared by using Pymol (20). (C) is based on a figure from Hall et al. (29). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).