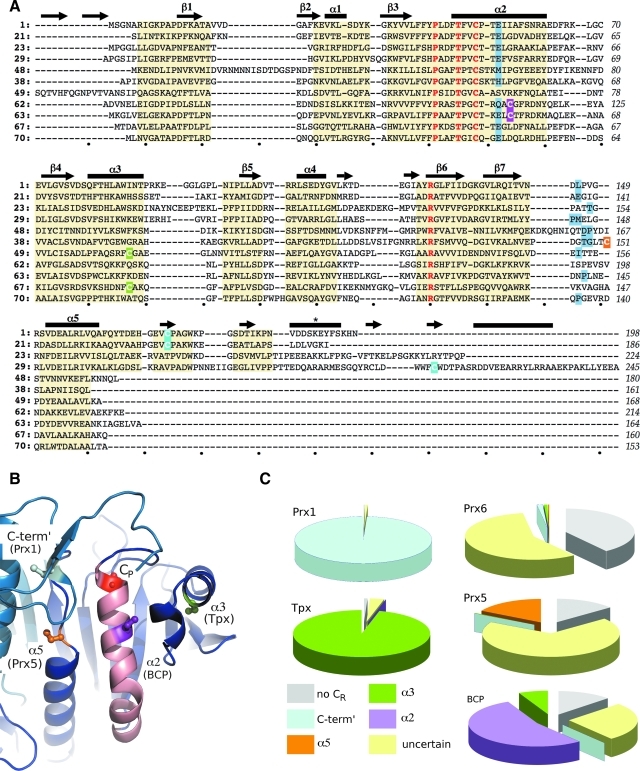
FIG. 4.
Variations in Prx sequences. (A) Structure-based sequence alignment of representative Prxs. Residues that have a common main-chain path among all Prxs are highlighted by a yellow background. Secondary structure elements are indicated above the alignment with the common-core Prx elements labeled as in Fig. 2, and other elements present in only some Prxs are shown but not labeled. The four residues conserved in all Prxs are colored red, and the CR position of each 2-Cys Prx is highlighted by a purple, green, orange, or cyan background for a CR placed in α2, α3, α5, or the C-terminus, respectively (B). *The YF-motif helix associated with some Prxs sensitive to overoxidation. Residues involved in backbone-mediated passing chain stabilization of the conserved Arg are given a blue background; in one case, Asp 163 of PfAOP (underlined residue in line 5 sequence) stabilizes the Arg via its side chain. Structures are referenced by index number from Table 1 and include a sensitive Prx1 (1), a robust Prx1 (21), a 1-Cys Prx6 (23), a 2-Cys Prx6 (29), a 1-Cys Prx5 (49), a 2-Cys Prx5 (38), a Tpx (50), a 1-Cys BCP (63, monomeric), a 2-Cys BCP (64, CR in α2, dimeric), a 2-Cys BCP (68, CR in α3, monomeric), and an AhpE (71). The last residue of each line is numbered and dots below the alignment mark every 10 spaces. (B) The four prototypical locations for the CR [colored as in (A) and labeled by location and the subfamily it is commonly associated with] are mapped onto a composite structure based on StAhpC (entry 21 in Table 1). The conserved CP (red) is also shown. The two chains of the B-type dimer are colored in dark and light blue, and helix α2 is colored pink. (C) Pie charts based on ∼3,500 Prx sequences showing the frequency at which the CR is in a given location for each subfamily. Wedges are colored by CR position consistent with (A) and (B), by using the notation in (B): no CR (gray), C-term′ (cyan), α5 (orange), α3 (green), α2 (purple), and uncertain (pale yellow). The exact positions are defined as follows: C-term′ aligns with residue 172 in HsPrxII (entry 1 in Table 1); α5 aligns with residue 151 (or −2 residues) in HsPrxV (entry 38 in Table 1); α3 aligns with residue 95 in EcTpx (entry 50 in Table 1); and α2 aligns with residue 112 in ScnTPx (entry 63 in Table 1). Sequences marked “uncertain” have additional Cys residues present, but none aligns exactly with one of the known locations. (B) was prepared by using Pymol (20). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).