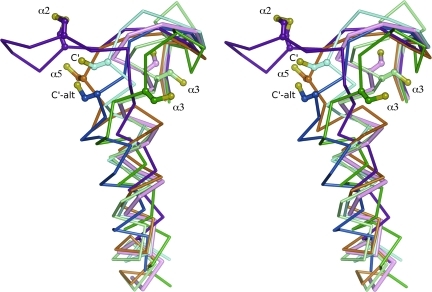
FIG. 5.
Local unfolding changes the conformation of α2 and the CP-loop. Comparison of the canonic FF structure (pink, entry 1 in Table 1) with LU representatives from each subfamily shows that the LU conformations of the CP-loop and helix α2 vary by subfamily. Shown in stereo and viewed as in Fig. 2B are the LU conformations of a Prx1 (light blue, entry 17 and dark blue, entry 15 in Table 1), a Prx5 (orange, entry 44 in Table 1), a Tpx (dark green, entry 53 in Table 1), an α2-BCP (purple, entry 64 in Table 1), and an α3-BCP (pale green, entry 69 in Table 1). Labels indicate the location of the CR (α2, α3, and α5, as in Fig. 4; C′ and C′-alt for the CR near the C-terminus as in Fig. 4 with “alt” for the ∼8-degree shift from the canonic conformation, as described). No LU example is given from the Prx6 or AhpE subfamilies. The CP residue in each structure is shown as ball-and-stick, with the sulfur atom colored yellow. The LU structures are all disulfide-bonded forms, even though the CR is shown only for the α2-BCP example. Figure was prepared by using Pymol (20). (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).