Abstract
Hormones mediate major physiological and behavioural components of the reproductive phenotype of individuals. To understand basic evolutionary processes in the hormonal regulation of reproductive traits, we need to know whether, and during which reproductive phases, individual variation in hormone concentrations relates to fitness in natural populations. We related circulating concentrations of prolactin and corticosterone to parental behaviour and reproductive success during both the pre-breeding and the chick-rearing stages in both individuals of pairs of free-living house sparrows, Passer domesticus. Prolactin and baseline corticosterone concentrations in pre-breeding females, and prolactin concentrations in pre-breeding males, predicted total number of fledglings. When the strong effect of lay date on total fledgling number was corrected for, only pre-breeding baseline corticosterone, but not prolactin, was negatively correlated with the reproductive success of females. During the breeding season, nestling provisioning rates of both sexes were negatively correlated with stress-induced corticosterone levels. Lastly, individuals of both sexes with low baseline corticosterone before and high baseline corticosterone during breeding raised the most offspring, suggesting that either the plasticity of this trait contributes to reproductive success or that high parental effort leads to increased hormone concentrations. Thus hormone concentrations both before and during breeding, as well as their seasonal dynamics, predict reproductive success, suggesting that individual variation in absolute concentrations and in plasticity is functionally significant, and, if heritable, may be a target of selection.
Keywords: stress, corticosterone, prolactin, Passer domesticus, parental investment
1. Introduction
Hormones regulate many aspects of an individual's phenotype, including various physiological and behavioural traits [1]. A full understanding of the evolution of fitness-relevant traits such as reproductive investment, therefore, requires a corresponding knowledge of the evolution of the endocrine mechanisms that control the expression of the phenotype [2–4]. One important component of studies in evolutionary physiology is heritable individual variation, especially in relation to individual fitness [5–7], as it is the raw material of selection [7–9]. Furthermore, knowledge of the dynamics of endocrine signalling in relation to the reproductive investment of individuals will increase our understanding of reproductive decision-making and life-history trade-offs (e.g. [6,10–12]).
Recent studies have demonstrated relationships between individual variation in circulating concentrations of hormones, behaviour and fitness during the breeding phase. For example, individual variation in plasma testosterone concentrations relates to male alternative reproductive strategies, territorial behaviour, paternal behaviour, reproductive success and survival in several vertebrates (e.g. [9,13–15]). Individual variation in plasma prolactin (Prl) concentrations correlates with nestling provisioning rates in birds [16,17] and with alternative male reproductive tactics in mammals [18]. In birds, individual variation in baseline concentrations of corticosterone (Cort0) correlates with parental care, timing of breeding and reproductive success, although the direction of the relationship appears to be species-specific and dependent on sex and reproductive stage [5,19–22] (see also [23]). Furthermore, stress-induced concentrations of corticosterone (maxCort) tend to show a negative relationship with reproductive behaviour [24,25].
These studies suggest that correlations between individual variation in concentrations of single hormones and reproductive performance during the breeding season are functionally significant [20,26,27]. However, they do not take into account that seasonal changes in the concentrations of two or more hormones may have interactive effects on reproductive performance. Furthermore, major decisions about reproductive investment often are made during the pre-breeding season, where we know much less about the relationship between hormones and reproductive phenotype. For example, in birds, lay date (a trait often closely linked with reproductive fitness [28]) is set before the start of the breeding season [29,30]. To our knowledge only two studies have examined the relationship between the natural variation in concentration of circulating hormones during the pre-breeding season and subsequent reproductive investment. In one of these studies, female marine iguanas (Amblyrhynchus cristatus) with low Cort0 and maxCort during the pre-breeding season were more likely to breed that year than those with high Cort0 and maxCort [31], while in the second study female snow petrels (Pagrodroma nivea) with elevated pre-breeding Cort0 had a high probability of skipping breeding that year [32]. Additionally, experimental treatment of female side-blotched lizards (Uta stansburiana) with Cort prior to the breeding season altered their tendency to reproduce, although in opposite directions depending on the reproductive strategy/morph of individuals [33].
Here, we determined whether Prl, Cort0 and maxCort of individuals measured during both the pre-breeding and the breeding season are related to individual differences in reproductive investment and success in breeding pairs of free-living house sparrow (Passer domesticus). House sparrows show marked individual variation in number of clutches laid per season, parental feeding rates and juvenile recruitment rates [34]. We focused on Cort and Prl as interactive mediators of reproductive decisions and trade-offs in light of their opposing actions on reproductive investment [19,24–26,35]. In birds, Prl secretion is stimulated by increasing photoperiods [36], with further increases at the onset of incubation [37]. Prl promotes parental care, thereby modulating the seasonal adjustments of reproductive effort [35,38]. Cort0 typically increases as an animal works harder, acting as a metabolic hormone by supporting energetically demanding processes (e.g. [39,40]). Cort concentrations can increase within 3 min when an individual experiences adverse conditions, and then typically shut down non-essential processes such as reproduction to promote survival functions [40–42].
In the current study, we first determined whether individuals have consistent hormone concentrations by calculating repeatabilities for pre-breeding and breeding season hormone concentrations. We also examined the level of correlation in hormone concentrations between members of a pair. Second, to establish whether variations in hormone concentrations relate to fitness we determined whether hormonal traits obtained during the pre-breeding and the breeding season were related to the reproductive success of an individual. Third, during the parental phase we determined the relationship between hormone concentrations and behavioural measures of parental investment such as nestling feeding rates.
2. Methods
(a). Study species and behavioural observations
We carried out the study between March and August 2008 on a free-living population of house sparrows that bred in nest-boxes of two large barns at a farm co-op in Belle Mead, NJ, USA (40°28′ N, 74°39′ W). We captured adults in mist nets, and, upon first capture, we individually marked them with a numbered aluminium ring and a unique combination of coloured leg bands. We monitored nests daily to determine laying dates, clutch sizes and number of hatchlings. Parental food provisioning rate (hereafter termed ‘feeding rate’) was determined for each individual by continuous scan observations [43] from a central location (about 100 m from nests) from 0700 to 0800 h during days 11 or 12 of the nestling phase of the first clutch of each pair. Scans were made on sunny days when there were no detectable disturbances nearby. House sparrows are sexually dimorphic and easily distinguished [44]. We captured and blood-sampled 49 adult birds on 9 March (before the breeding season) with mist nets, 24 days before the first eggs were laid in the study population. Of these, 20 females and 20 males were pair-bonded and nested in nest-boxes inside the barn. We recaptured both members of each pair during the breeding season using manually triggered spring-loaded traps, shutting the entrance hole as they entered the nest to feed 8–10-day-old nestlings of their first clutch (between 27 April and 2 June). Pairs remained bonded for the duration of the breeding season. We searched the field site (51 ha) every other day between March and August, and every week between August and late October, for additional nests. This sedentary population of house sparrows relies on the study site for food and available nesting habitat, making it unlikely that additional nests outside the core study area were not found. Nest-boxes were located at a height of about 10 m inside enclosed storage barns, and there was no nest predation.
(b). Measurement of hormone concentrations
Immediately after each capture, a blood sample (total less than 200 µl) was collected from each individual from the brachial vein by venipuncture for the determination of Cort0 and Prl, and the time required to do so (from hitting the mist net or springing the nest trap to completing collection) was recorded. The first 70–100 µl of blood collected was used for measurement of Cort0 (mean handling time: 2.0 ± 0.2 min; maximum: 3.3 min), while the second 70–100 µl was collected for Prl determination (mean handling time: 3.5 ± 0.3 min; maximum: 6.03 min). Cort0 and Prl concentrations in these blood samples were not related to handling time (Cort0: r = −0.22, p = 0.20, n = 80; Prl: r = −0.14, p = 0.31, n = 80). We then used a standard capture–handling–restraint protocol (see [41]) to determine maxCort concentrations. For this, following the initial collection of blood samples, we placed each bird in a cloth bag and collected a final blood sample (less than 70 µl) 30 min later. We chose 30 min as the time for the final sample because previous studies on this species have shown that Cort concentrations reach the maximum values at this time [45]. We then measured tarsus length (±0.1 mm) and body mass (±0.1 g) before releasing the birds at the site of capture. The blood samples were kept on ice and centrifuged (3000 rpm/1276 g, 10 min) within 3 h, and the plasma was separated and stored at −20°C for hormone analyses.
(c). Hormone assays
Circulating Cort concentrations were determined in a single radioimmunoassay [46]. Cort antibody was purchased from Esoterix Endocrinology, CA, USA. All samples were assayed in duplicate. Average recovery after extraction with dichloromethane of samples spiked with a small amount of radio-labelled hormone was 82.9 ± 1.8%; final concentrations were corrected for individual extraction efficiencies. The lower limit of detection of the assay was at 1.99 ng ml–1; the intra-assay coefficient of variation as estimated by taking replicate Cort standards of known concentrations through the entire assay procedure (one at low and one at medium concentration were included in the beginning and the end of the assay, respectively) was 13.6 per cent. Plasma Prl concentrations were determined using a direct recombinant-derived starling (Sturnus vulgaris) Prl radioimmunoassay [47]. Samples were assayed in duplicate when there was sufficient sample volume, but in most cases there was not. The reaction volume was 60 µl, comprising 20 µl of plasma sample or standard, 20 µl of primary rabbit antibody to starling Prl (1 : 24 000) and 20 µl of I125-labelled Prl (15 000 cpm). The primary antibody was precipitated to separate free and bound I125 label using 20 µl of donkey anti-rabbit precipitating serum and 20 µl of non-immune rabbit serum. All samples were measured in a single assay. The intra-assay coefficient of variation was 8.5 per cent; the minimum detectable dose was 1.0 ng ml–1, with a 50 per cent displacement at 12.14 ng ml–1.
(d). Data analysis
Data for both sexes were analysed separately to avoid pseudo-replication of data on fledgling numbers from the same nest/pair. Pre-breeding and breeding season data were also analysed separately. Data for total fledgling number followed a normal distribution (Shapiro–Wilk test: n = 40, z = 1.54, p > 0.07). We used multiple regression models to predict total fledgling numbers from the variables Cort0, maxCort and Prl concentration. Because lay date was highly correlated with total number of fledglings, we controlled for this by adding lay date into the model. We initially included all three hormonal traits in the model and then used backward elimination to remove any non-significant correlations. Body condition was calculated by using residuals from a linear regression of body mass against tarsus length and was included in all models to control for effects of body condition on reproductive success. We also ran all analyses with body mass and tarsus length included as separate covariates in the models. Both methods gave similar results (example of one model: r2 = 0.9258 including body condition and r2 = 0.9292 with body mass and tarsus length), and we opted to include body condition as calculated from residuals as above in our models because in our dataset body mass and tarsus length were linearly correlated (r = 0.61, p = 0.0008). Omitting body condition from our models entirely gave very similar results to the ones reported below. Changes in hormone concentrations were calculated as breeding season minus pre-breeding season concentrations, and we used backward elimination to generate the best model that predicted total fledgling numbers from the changes in hormone concentrations. The ideal statistical approach to analyse our dataset would have been to include all variables, both sexes and both seasons into one single model to determine the relative importance of each parameter. However, our sample sizes precluded such models, and therefore necessitated the use of separate models for each sex and breeding stage. Pearson's correlations were used to test whether the behaviours and hormone concentrations in adult pairs were correlated. Repeatability of hormone concentrations between pre-breeding to breeding seasons were calculated from between- and within-group variances derived from one-way ANOVAs according to Lessells & Boag [48]. Analyses were performed using STATA 9.0 (College Station, TX, USA). Sample sizes for both females and males in both seasons were n = 20. Data are given as means ± 1 s.e.m.
3. Results
(a). Reproductive characteristics and individual hormone consistencies
Pairs began displaying courtship behaviour in February and the first egg was laid on 2 April. The mean first clutch initiation date for pairs that laid three clutches was 6 April ±1 day (n = 8); for pairs that laid two clutches it was 20 April ±3 days (n = 8); and for pairs that laid one clutch it was 14 May ±3 days (n = 4). Early-laying females produced a greater total number of clutches (and thus total number of eggs) during the season (r = −0.91, p < 0.0001, n = 20). Average clutch size was 4.56 ± 0.72 (range 4–6), with 96.5 per cent of the eggs hatching. Mean clutch sizes of females that laid different number of clutches did not differ (χ2 = 4.42, d.f. = 2, p = 0.11), so the main difference in reproductive output was in the number of clutches laid.
Prl concentrations in the same individual were repeatable (variation between pre-breeding and breeding concentrations within an individual was lower than variation among individuals) in males (r = 0.65, d.f. = 19, F = 2.55, p = 0.02) and in females (r = 0.68, d.f. = 19, F = 6.24, p < 0.0001) from the pre-breeding to the nestling stages of the reproductive cycle. Cort0 was repeatable neither in males (r = 0.04, d.f. = 19, F = 0.28, p = 0.89) nor in females (r = 0.04, d.f. = 19, F = 0.28, p = 0.88) from the pre-breeding to the nestling stages. MaxCort was repeatable neither in females (r = 0.08, d.f. = 19, F = 0.86, p = 0.62) nor in males (r = 0.23, d.f. = 19, F = 1.56, p = 0.16).
Hormone levels of the members of a pair were positively correlated with each other, both before the breeding season (Prl: r = 0.78, p < 0.0001; Cort0: r = 0.77, p < 0.0001; maxCort: r = 0.47, p = 0.01) and during the breeding season (Prl: r = 0.53, p = 0.003; Cort0: r = 0.50, p = 0.004; maxCort: r = 0.49, p = 0.008). Feeding rates were also positively correlated between members of a pair (r = 0.77, n = 20, p < 0.0001).
(b). Hormones and reproductive success
Pre-breeding body condition was negatively correlated with pre-breeding Cort0 (females: r = −0.47, p = 0.035; males: r = −0.53, p = 0.015) and positively correlated with breeding Cort0 levels (females: r = 0.66, p = 0.0017; males: r = 0.48, p = 0.034).
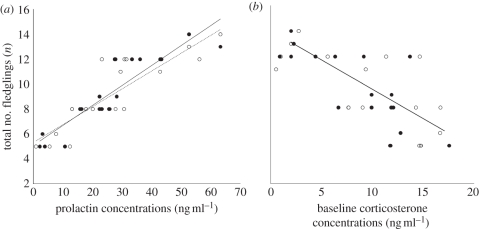
In females, both Cort0 and Prl concentrations, and in males Prl but not Cort0, during the pre-breeding season predicted total number of fledglings for the entire season (overall model: females: F = 61.20, d.f. = 5, p < 0.00001, r2 = 0.93; males: d.f. = 3, F = 27.62, p < 0.00001, r2 = 0.81; see table 1). Females with low Cort0 and high Prl concentrations during the pre-breeding season fledged the most offspring, while in males only high pre-breeding Prl was associated with increased reproductive success (figure 1).
Table 1.
Results from multiple regression model to predict total number of fledglings from variables measured during the pre-breeding season. Numbers in bold indicate values are significant at the 0.05 level.
| coefficient | s.e. | t | partial r | p | |
|---|---|---|---|---|---|
| females | |||||
| lay date | −1.772 | 0.031 | −5.88 | −0.78 | 0.0001 |
| cort0 | −1.098 | 0.032 | −4.18 | −0.71 | 0.001 |
| prolactin | 0.941 | 0.064 | 2.40 | 0.61 | 0.030 |
| body condition | −2.600 | 0.565 | −0.52 | −0.25 | 0.609 |
| males | |||||
| lay date | −2.230 | 0.558 | −3.64 | −0.65 | 0.002 |
| prolactin | 1.026 | 0.448 | 2.23 | 0.52 | 0.040 |
| body condition | 7.177 | 9.966 | 0.78 | 0.09 | 0.447 |
Figure 1.
Relationships between total number of fledglings produced by individual birds during the breeding season and (a) pre-breeding prolactin concentrations and (b) pre-breeding baseline corticosterone concentrations (ng ml–1). Females: closed symbols and solid line of best fit; males: open symbols and dashed line of best fit.
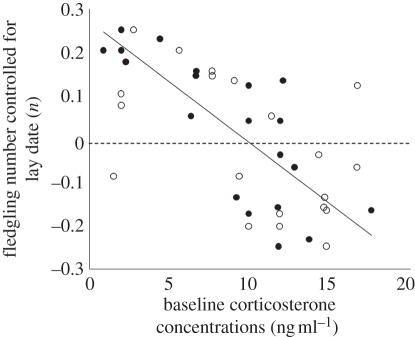
As indicated by bivariate correlations, lay date was the strongest predictor of the number of fledglings (pairs: r = −0.86, p < 0.0001). Bivariate correlations showed that Prl was more closely associated with lay date than Cort0 (Prl with lay date: females: r = −0.69, p < 0.0007; males: r = −0.70, p = 0.0006; Cort0 with lay date: females: r = 0.39, p = 0.093; males: r = 0.45, p = 0.044). To understand which hormonal traits are associated with lay date (and thereby with fitness) as opposed to traits that contribute to fitness independently of lay date, we computed the residuals from a regression of the number of fledglings and lay date. This fitness variable was thereby ‘corrected’ for lay date and included in a modified version of the multiple regression model. Using this model, it was found in females that hormones contributed to explaining the variance of the corrected number of fledglings (F = 4.00, p = 0.027, d.f. = 4, r2 = 0.32), whereas in males hormones had no significant effect on this variance (F = 2.22, p = 0.13, r2 = 0.16; table 2). In females, pre-breeding Cort0 was negatively correlated with the corrected number of fledglings; that is, females that had the largest fledgling numbers regardless of lay date had the lowest pre-breeding Cort0 (F = 6.82, r2 = 0.45, p = 0.006; figure 2).
Table 2.
Results from multiple regression models to predict total number of fledglings controlled for lay date from variables measured during the pre-breeding season. Number in bold indicates value is significant at the 0.05 level.
| coefficient | s.e. | t | partial r | p | |
|---|---|---|---|---|---|
| females | |||||
| cort0 | −0.116 | 0.042 | −2.74 | −0.47 | 0.014 |
| prolactin | 0.037 | 0.044 | 0.84 | 0.11 | 0.421 |
| body condition | 0.268 | 0.725 | 0.37 | 0.12 | 0.717 |
| males | |||||
| cort0 | −0.051 | 0.046 | −1.08 | −0.26 | 0.289 |
| prolactin | 0.062 | 0.042 | 1.42 | 0.29 | 0.177 |
| body condition | −0.426 | 1.134 | −0.43 | −0.12 | 0.581 |
Figure 2.
Correlation between residuals of total fledgling number (controlled for lay date) and pre-breeding baseline corticosterone concentrations (ng ml–1). Individual females above the dotted zero line had more fledglings and lower pre-breeding baseline corticosterone concentrations than females below the dotted zero line regardless of lay date. Females: closed symbols and solid line of best fit; males: open symbols.
Hormone concentrations during feeding of the first clutch significantly predicted total number of fledglings (females: F = 32.52, d.f. = 3, r2 = 0.83, p < 0.0001; males: F = 24.13, d.f. = 3, r2 = 0.79, p < 0.0001). However, only Prl concentrations were significant and thus included in this model: individuals with the highest Prl while rearing their first clutch fledged the most young during the entire breeding season (females: r2 = 0.74, t = 2.75, p = 0.014; males: r2 = 0.81, t = 2.45, p = 0.026).
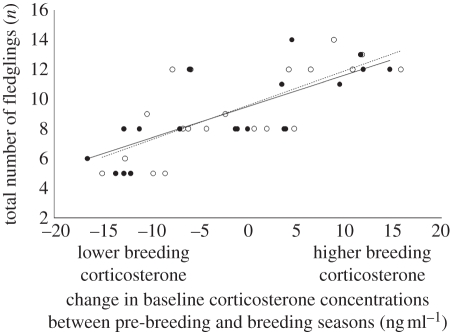
The relationship between Cort0 and total number of fledglings changed between the pre-breeding and breeding season in both sexes. Moreover, the direction of the change in Cort0 was important for fitness: individuals that had low Cort0 during pre-breeding but high Cort0 during the breeding season fledged more young during the entire season than individuals that had high pre-breeding Cort0 and low breeding Cort0 (females: F = 5.65, p = 0.01, r2 = 0.40; males: F = 8.47, p = 0.003, r2 = 0.50; figure 3).
Figure 3.
The direction of the change in baseline corticosterone concentrations (breeding-pre-breeding baseline corticosterone; ng ml–1) is related to total number of fledglings (n). Individuals with low pre-breeding and high breeding baseline corticosterone had the highest reproductive success. Females: closed symbols and solid line of best fit; males: open symbols and dashed line of best fit.
(c). Hormones and parental behaviour
Feeding rates per hour and feeding rates per hour per young were positively correlated (r = 0.87, n = 40, p < 0.0001) because for the first brood 80 per cent of the observed pairs fledged five young. Thus, we opted to use feeding rate per hour to quantify parental investment for each adult. Feeding rates of nestlings from the first clutch were predicted by breeding maxCort levels in both females (F = 26.73, d.f. = 1, r2 = 0.58, p < 0.0001) and males (F = 17.74, d.f. = 2, r2 = 0.64, p < 0.0001), with individuals that reached the highest maxCort concentrations showing lower feeding rates (figure 4). In initial bivariate analyses, Prl correlated positively with feeding rates (females: r = 0.63, p = 0.003; males: r = 0.68, p = 0.001), but Prl was not a significant variable when included together with maxCort in the above model.
Figure 4.
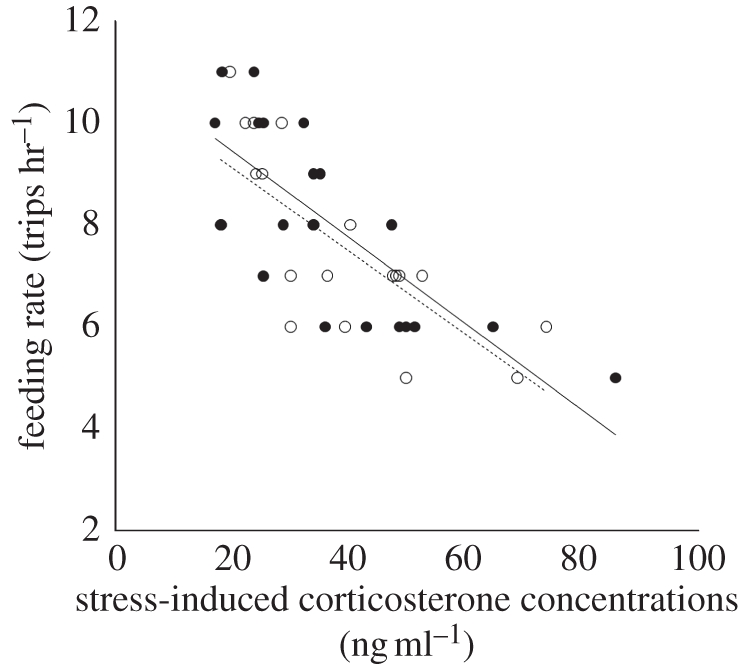
Stress-induced corticosterone concentrations (ng ml–1) during the breeding season were negatively correlated with parental provisioning rates (number of trips hr−1). Females: closed symbols and solid line of best fit; males: open symbols and dashed line of best fit.
4. Discussion
This study shows that individual variation in baseline corticosterone (Cort0) concentrations several weeks before first eggs were laid and in prolactin (Prl) during the parental phase of the first clutch predicted individual reproductive success during the entire season. Furthermore, not only were absolute hormone concentrations important in determining fitness, seasonal dynamics in Cort0 concentrations also predicted reproductive success.
(a). Hormones and reproductive success
Individuals of both sexes with the highest pre-breeding Prl concentrations had the greatest total reproductive output (figure 1a). However, Prl appeared to be most strongly related to lay date, which in turn is a strong determinant of overall reproductive success in a season [49,50]. This relationship could be caused by several processes. Prl increases in response to increasing day length prior to the breeding season [51], and birds laying early clutches might have a seasonally accelerated photoperiodic induction of Prl secretion. Alternatively, at the time of sampling, individuals with early lay dates might have been at a more advanced stage of preparedness for breeding, and Prl secretion may have been stimulated to a greater extent, for example, by the presence of a nest [37]. In American kestrels (Falco sparverius) and pheasants (Phasianus colchicus), Prl concentrations rise with proximity to the onset of incubation [52,53]. Our data do not allow us to determine whether individual variation in pre-breeding concentrations of Prl reflect genetic differences that also determine lay date, whether Prl is causally involved in determining the decision of when to lay or whether Prl concentrations were the consequence of reproductive decisions having already been made. Experimental approaches such as manipulation of lay date, clutch size or Prl concentrations will be required to distinguish between these possibilities.
When we controlled total numbers of young fledged for lay date in the present study, the residual variance for females was only explained by pre-breeding Cort0, in that females with low pre-breeding Cort0 had higher total fledgling numbers during the breeding season (figure 2). Pre-breeding Cort0 appeared inversely related to female quality, as females with lower Cort0 had higher body condition and higher subsequent reproductive output, irrespective of lay date. This finding is consistent with that of Vitousek et al. [31], in which female reptiles with lower pre-breeding Cort0 also had higher reproductive output during the breeding season. In our study, we could not determine age, experience or genetic make-up of individuals, and hence were not able to quantify the potential importance of those factors on reproductive performance [54,55]. However, among birds that laid the same number of clutches there was ample individual variation in reproductive success (figure 1), of which pre-breeding Cort0 explained a considerable part.
Even more intriguing is the finding that individuals with low pre-breeding but high breeding Cort0 concentrations raised more fledglings during the entire breeding season than individuals with a similar degree of plasticity but in the opposite direction (high pre-breeding and low breeding Cort0; figure 3). This suggests that a certain type of plasticity (specifically an up-regulation) of Cort0 in the course of the reproductive season is an important component of reproductive success. An alternative hypothesis is that birds with low Cort0 were more likely to initiate more clutches, and the increased parental effort associated with the act of raising more nestlings is what is driving the Cort0 increase. Increased Cort0 during the breeding season might support the challenges of provisioning a brood by promoting the utilization of resources to address high energetic demands [56,57]. Indeed, an up-regulation of Cort0 was also observed in female tree-swallows (Tachycineta bicolor) with higher reproductive success from incubation to chick rearing [39]. Furthermore, in white-crowned sparrows (Zonotrichia leucophrys), females with lowest Cort0 during incubation had the highest yearly reproductive success (although this was not observed in males [21]).
MaxCort was not related to reproductive success during the pre-breeding nor the breeding season when included together with Cort0 and Prl in statistical tests (although it was related to parental behaviour; figure 4). This suggests that the functional role of maxCort differs from that of Cort0 [40,58,59]. In the current study, maxCort appears unrelated to reproductive decision-making in the pre-breeding period and instead may be a modulator of reproductive effort once breeding is under way (see below). Instead, Prl concentrations in both sexes during the breeding season (while raising the young of their first brood) were positively associated with the total number of fledglings produced during that year. This could be because birds with high prolactin are more likely to raise subsequent broods. In single-brooded starlings, prolactin concentrations decreased in both sexes after the parental stage [60], whereas in double-brooded song sparrows (Melospiza melodia) prolactin remained high between the two broods and did not decrease until after the second parental stage [61].
Although clutch numbers and sizes are under female control, hormone concentrations of males caught during the pre-breeding season correlated with those of their female partner, raising the possibility that individuals of similar quality and/or reproductive state may pair-bond associatively (e.g. [62]). Alternatively, hormone profiles of males and females might become more correlated after pairing.
(b). Hormones and parental behaviour
In the current study, maxCort concentrations during the breeding season showed an inverse relationship with feeding visits to the nest: individuals of both sexes that reached lower maxCort concentrations during a standardized capture–restraint protocol showed higher nestling provisioning rates than individuals that reached higher maxCort concentrations. This is in agreement with other studies showing that maxCort during the breeding season in individuals of different species correlates inversely with parental effort [26,63,64]. Further experiments are needed to establish whether individuals with lower maxCort concentrations actively suppress their stress response or whether their stress response is lower because of their state and/or reproductive strategy (see [24,65]). In other studies, Prl correlated with nestling feeding rate when measured on its own (see [35]), whereas in our study, when measured together with maxCort, the latter hormone was more important in explaining parental effort. This highlights the importance of studying multiple endocrine signals in conjunction to fully understand how hormones mediate behaviour.
5. Conclusion
This study suggests that in free-living house sparrows, circulating hormone concentrations during the pre-breeding and the breeding season can translate into individual variation in reproductive performance upon which selection could act (e.g. [66]). For Cort0, both absolute levels within a reproductive stage and seasonal plasticity were positively correlated with reproductive success. Whether and to what degree absolute hormone concentrations or plasticity in endocrine responses is heritable, and thus amenable to selection, remains to be established. Heritabilities of Cort0 and Prl concentrations are still unclear, although it is tantalizing that Prl concentrations in this study were consistent within individuals and that maxCort concentrations in birds appear to have a heritable component [67,68]. Furthermore, plastic physiological responses, which can be equated with reaction norms, can be heritable (e.g. [69,70]). It will be important in future studies to determine the degree of among-year repeatability and heritability in hormone concentrations, or their plasticity, to determine the evolutionary potential of hormonal traits. Further studies are also required to determine whether the reproductive success of both males and females is directly related to their own hormone concentrations, or whether there are indirect effects through the mate's phenotype. Finally, although we found relationships between hormonal traits and reproductive success in males, we could not determine the rate of extra pair fertilization (EPF) in our population and thus the real reproductive success for each individual male. The EPF rate in house sparrows in a population can be around 20 per cent [71], which may affect the direction and strength of the relationship between hormones and reproductive success in males. Nevertheless, the demonstration here of rather tight relationships between individual variation in hormone concentrations and reproductive performance represents an important advance in our understanding of evolutionary endocrinology.
Acknowledgements
All procedures used in this study were approved by the Princeton University Institutional Animal Care and Use Committee.
Many thanks to J. Adelman, I. Bisson, S. Cordoba, M. Echeverry, K. Spoelstra and A. Shaw for field assistance. A. Baugh, M. Echeverry, T. Greives, G. Burness, D. Rubenstein, two anonymous reviewers and the Associate Editor provided insightful comments on earlier drafts of this paper. We are grateful to the Belle Mead Co-op for the use of their facilities and their generous accommodations during the catching process. This project was supported by Sigma Xi Grant-in-Aid of Research, Princeton's Department of Ecology and Evolutionary Biology's summer grant, and an NSF graduate research fellowship to J.Q.O. by the Max Planck Society to M.H. and by the Roslin Institute, University of Edinburgh to P.J.S.
References
- 1.Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Ketterson E. D., Nolan V. J. 1992. Hormones and life histories: an integrative approach. Am. Nat. 140, S33–S62 10.1086/285396 (doi:10.1086/285396) [DOI] [PubMed] [Google Scholar]
- 3.Wingfield J. C., Maney D. L., Breuner C. W., Jacobs J. D., Lynn S., Ramenofsky M., Richardson R. D. 1998. Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206 [Google Scholar]
- 4.Zera A. J., Harshman L. G., Williams T. D. 2007. Evolutionary endocrinology: the developing synthesis between endocrinology and evolutionary genetics. Ann. Rev. Ecol. Evol. Syst. 38, 793–817 10.1146/annurev.ecolsys.38.091206.095615 (doi:10.1146/annurev.ecolsys.38.091206.095615) [DOI] [Google Scholar]
- 5.Bonier F., Martin P. R., Moore I. T., Wingfield J. C. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642 10.1016/j.tree.2009.04.013 (doi:10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 6.Sinervo B., Licht P. 1991. Hormonal and physiological control of clutch size, egg size, and egg shape in side-blotched lizards (Uta stansburiana)—constraints on the evolution of lizard life histories. J. Exper. Zool. 257, 252–264 10.1002/jez.1402570216 (doi:10.1002/jez.1402570216) [DOI] [Google Scholar]
- 7.Williams T. D. 2008. Individual variation in endocrine systems: moving beyond the ‘tyranny of the golden mean’. Phil. Trans. R. Soc. B 363, 1687–1698 10.1098/rstb.2007.0003 (doi:10.1098/rstb.2007.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett A. F. 1987. Interindividual variability: an underutilized resource. In New directions in ecological physiology (eds Feder M. E., Bennett A. F., Burggren W. W., Huey R. B.), pp. 147–169 Cambridge, UK: Cambridge University Press [Google Scholar]
- 9.Kempenaers B., Peters A., Foerster K. 2008. Sources of individual variation in plasma testosterone levels. Phil. Trans. R. Soc. B 363, 1711–1723 10.1098/rstb.2007.0001 (doi:10.1098/rstb.2007.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingemanse N. J., Edelaar P., Kempenaers B. 2010. Why is there variation in baseline glucocorticoid levels? Trends Ecol. Evol. 25, 261–262 10.1016/j.tree.2010.01.008 (doi:10.1016/j.tree.2010.01.008) [DOI] [PubMed] [Google Scholar]
- 11.McGlothlin J. W., Whittaker D. J., Schrock S. E., Gerlach N. M., Jawor J. M., Snajdr E. A., Ketterson E. D. 2010. Natural selection on testosterone production in a wild songbird population. Am. Nat. 175, 687–701 10.1086/652469 (doi:10.1086/652469) [DOI] [PubMed] [Google Scholar]
- 12.Zera A. J., Harshman L. G. 2001. The physiology of life history trade-offs in animals. Ann. Rev. Ecol. Syst. 32, 95. 10.1146/annurev.ecolsys.32.081501.114006 (doi:10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 13.Reed W. L., Clark M. E., Parker P. G., Raouf S. A., Arguedas N., Monk D. S., Snajdr E., Nolan V., Ketterson E. D. 2006. Physiological effects on demography: a long-term experimental study of testosterone's effects on fitness. Am. Nat. 167, 667–683 10.1086/503054 (doi:10.1086/503054) [DOI] [PubMed] [Google Scholar]
- 14.Sinervo B., Miles D. B., Frankino W. A., Klukowski M., DeNardo D. F. 2000. Testosterone, endurance, and Darwinian fitness: natural and sexual selection on the physiological bases of alternative male behaviors in side-blotched lizards. Horm. Behav. 38, 222–233 10.1006/hbeh.2000.1622 (doi:10.1006/hbeh.2000.1622) [DOI] [PubMed] [Google Scholar]
- 15.Trainor B. C., Marler C. A. 2001. Testosterone, paternal behavior, and aggression in the monogamous California mouse, Peromyscus californicus. Horm. Behav. 40, 32–42 10.1006/hbeh.2001.1652 (doi:10.1006/hbeh.2001.1652) [DOI] [PubMed] [Google Scholar]
- 16.Badyaev A. V., Duckworth R. A. 2005. Evolution of plasticity in hormonally-integrated parental tactics. In Functional avian endocrinology (eds Dawson A., Sharp P. J.), pp. 375–386 New Delhi, India: Narosa Publishing House [Google Scholar]
- 17.Chastel O., Lacroix A., Weimerskirch H., Gabrielsen G. W. 2005. Modulation of prolactin but not corticosterone responses to stress in relation to parental effort in a long-lived bird. Horm. Behav. 47, 459–466 10.1016/j.yhbeh.2004.10.009 (doi:10.1016/j.yhbeh.2004.10.009) [DOI] [PubMed] [Google Scholar]
- 18.Schradin C. 2008. Differences in prolactin levels between three alternative male reproductive tactics in striped mice (Rhabdomys pumilio). Proc. R. Soc. B 275, 1047–1052 10.1098/rspb.2008.0004 (doi:10.1098/rspb.2008.0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angelier F., Chastel O. 2009. Stress, prolactin and parental investment in birds: a review. Gen. Comp. Endocrinol. 163, 142–148 10.1016/j.ygcen.2009.03.028 (doi:10.1016/j.ygcen.2009.03.028) [DOI] [PubMed] [Google Scholar]
- 20.Angelier F., Wingfield J. C., Weimerskirch H., Chastel O. 2010. Hormonal correlates of individual quality in a long-lived bird: a test of the ‘corticosterone–fitness hypothesis’. Biol. Lett. 6, 846–849 10.1098/rsbl.2010.0376 (doi:10.1098/rsbl.2010.0376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonier F., Martin P. R., Sheldon K. S., Jensen J. P., Foltz S. L., Wingfield J. C. 2007. Sex-specific consequences of life in the city. Behav. Ecol. 18, 121–129 10.1093/beheco/arl050 (doi:10.1093/beheco/arl050) [DOI] [Google Scholar]
- 22.Schoech S. J., Rensel M. A., Bridge E. S., Boughton R. K., Wilcoxen T. E. 2009. Environment, glucocorticoids, and the timing of reproduction. Gen. Comp. Endocrinol. 163, 201–207 10.1016/j.ygcen.2008.09.009 (doi:10.1016/j.ygcen.2008.09.009) [DOI] [PubMed] [Google Scholar]
- 23.Foerster S., Monfort S. L. 2010. Fecal glucocorticoids as indicators of metabolic stress in female Sykes' monkeys (Cercopithecus mitis albogularis). Horm. Behav. 58, 685–697 10.1016/j.yhbeh.2010.06.002 (doi:10.1016/j.yhbeh.2010.06.002) [DOI] [PubMed] [Google Scholar]
- 24.Lendvai A. Z., Giraudeau M., Chastel O. 2007. Reproduction and modulation of the stress response: an experimental test in the house sparrow. Proc. R. Soc. B 274, 391–397 10.1098/rspb.2006.3735 (doi:10.1098/rspb.2006.3735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Love O. P., Breuner C. W., Vezina F., Williams T. D. 2004. Mediation of a corticosterone-induced reproductive conflict. Horm. Behav. 46, 59–65 10.1016/j.yhbeh.2004.02.001 (doi:10.1016/j.yhbeh.2004.02.001) [DOI] [PubMed] [Google Scholar]
- 26.Angelier F., Clement-Chastel C., Welcker J., Gabrielsen G. W., Chastel O. 2009. How does corticosterone affect parental behaviour and reproductive success? A study of prolactin in black-legged kittiwakes. Funct. Ecol. 23, 784–793 10.1111/j.1365-2435.2009.01545.x (doi:10.1111/j.1365-2435.2009.01545.x) [DOI] [Google Scholar]
- 27.Silverin B., Arvidsson B., Wingfield J. 1997. The adrenocortical responses to stress in breeding Willow Warblers Phylloscopus trochilus in Sweden: effects of latitude and gender. Funct. Ecol. 11, 376–384 10.1046/j.1365-2435.1997.00097.x (doi:10.1046/j.1365-2435.1997.00097.x) [DOI] [Google Scholar]
- 28.Horak P., Mand R., Ots I. 1997. Identifying targets of selection: a multivariate analysis of reproductive traits in the Great Tit. Oikos 78, 592–600 10.2307/3545622 (doi:10.2307/3545622) [DOI] [Google Scholar]
- 29.Cresswell W., McCleery R. 2003. How Great Tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. J. Anim. Ecol. 72, 356–366 10.1046/j.1365-2656.2003.00701.x (doi:10.1046/j.1365-2656.2003.00701.x) [DOI] [Google Scholar]
- 30.Meijer T., Daan S., Hall M. 1990. Family planning in the kestrel (Falco tinnunculus): the proximate control of covariation of laying date and clutch size. Behav. 114, 117–136 10.1163/156853990X00077 (doi:10.1163/156853990X00077) [DOI] [Google Scholar]
- 31.Vitousek M. N., Mitchell M. A., Romero L. M., Awerman J., Wikelski M. 2010. To breed or not to breed: physiological correlates of reproductive status in a facultatively biennial iguanid. Horm. Behav. 57, 140–146 10.1016/j.yhbeh.2009.09.020 (doi:10.1016/j.yhbeh.2009.09.020) [DOI] [PubMed] [Google Scholar]
- 32.Goutte A., Antoine É., Weimerskirch H., Chastel O. 2010. Age and the timing of breeding in a long-lived bird: a role for stress hormones? Funct. Ecol. 24, 1007–1016 10.1111/j.1365-2435.2010.01712.x (doi:10.1111/j.1365-2435.2010.01712.x) [DOI] [Google Scholar]
- 33.Lancaster L. T., Hazard L. C., Clobert J., Sinervo B. R. 2008. Corticosterone manipulation reveals differences in hierarchical organization of multidimensional reproductive trade-offs in r-strategist and K-strategist females. J. Evol. Biol. 21, 556–565 10.1111/j.1420-9101.2007.01478.x (doi:10.1111/j.1420-9101.2007.01478.x) [DOI] [PubMed] [Google Scholar]
- 34.Ringsby T. H., Berge T., Saether B. E., Jensen H. 2009. Reproductive success and individual variation in feeding frequency of House Sparrows (Passer domesticus). J. Ornithol. 150, 469–481 10.1007/s10336-008-0365-z (doi:10.1007/s10336-008-0365-z) [DOI] [Google Scholar]
- 35.Buntin J. D. 1996. Neural and hormonal control of parental behavior in birds. In Parental care: evolution, mechanisms, and adaptive significance, vol. 25 (eds Rosenblatt J. S., Snowdon C. T.), pp. 161–213 Advances in the Study of Behavior. New York, NY: Academic Press Ltd [Google Scholar]
- 36.Sharp P. J., Dawson A., Lea R. E. 1998. Control of luteinizing hormone and prolactin secretion in birds. Comp. Biochem. Physiol. C 119, 275–282 10.1016/S0742-8413(98)00016-4 (doi:10.1016/S0742-8413(98)00016-4) [DOI] [PubMed] [Google Scholar]
- 37.Dawson A., Goldsmith A. R. 1985. Modulation of gonadotrophin and prolactin secretion by daylength and breeding behaviour in free-living starlings Sturnus vulgaris. J. Zool. Lond. A 206, 241–252 10.1111/j.1469-7998.1985.tb05648.x (doi:10.1111/j.1469-7998.1985.tb05648.x) [DOI] [Google Scholar]
- 38.Sockman K. W., Sharp P. J., Schwabl H. 2006. Orchestration of avian reproductive effort: an integration of the ultimate and proximate bases for flexibility in clutch size, incubation behaviour, and yolk androgen deposition. Biol. Rev. 81, 629–666 10.1017/S1464793106007147 (doi:10.1017/S1464793106007147) [DOI] [PubMed] [Google Scholar]
- 39.Bonier F., Moore I. T., Martin P. R., Robertson R. J. 2009. The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen. Comp. Endocrinol. 163, 208–213 10.1016/j.ygcen.2008.12.013 (doi:10.1016/j.ygcen.2008.12.013) [DOI] [PubMed] [Google Scholar]
- 40.Sapolsky R. M., Romero L. M., Munck A. U. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 10.1210/er.21.1.55 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 41.Wingfield J. C., Romero L. M. 2001. Adrenocortical responses to stress and their modulation in free-living vertebrates. In Handbook of physiology; section 7: the endocrine system; volume IV: coping with the environment: neural and endocrine mechanisms (eds McEwen B. S., Goodman H. M.), pp. 211–234 New York, NY: Oxford University Press [Google Scholar]
- 42.Wingfield J. C., Sapolsky R. M. 2003. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 15, 711–724 10.1046/j.1365-2826.2003.01033.x (doi:10.1046/j.1365-2826.2003.01033.x) [DOI] [PubMed] [Google Scholar]
- 43.Altmann J. 1974. Observational study of behaviour: sampling methods. Behaviour 49, 227–267 10.1163/156853974X00534 (doi:10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 44.Summers-Smith J. D. 1963. The house sparrow. London, UK: Collins [Google Scholar]
- 45.Breuner C. W., Orchinik M. 2001. Seasonal regulation of membrane and intracellular corticosteroid receptors in the house sparrow brain. J. Neuroendocrinol. 13, 412–420 10.1046/j.1365-2826.2001.00646.x (doi:10.1046/j.1365-2826.2001.00646.x) [DOI] [PubMed] [Google Scholar]
- 46.Gill S. A., Costa L. M., Hau M. 2008. Males of a single-brooded tropical bird species do not show increases in testosterone during social challenges. Horm. Behav. 54, 115–124 10.1016/j.yhbeh.2008.02.003 (doi:10.1016/j.yhbeh.2008.02.003) [DOI] [PubMed] [Google Scholar]
- 47.Bentley G. E., Goldsmith A. R., Dawson A., Glennie L. M., Talbot R. T., Sharp P. J. 1997. Photorefractoriness in European starlings (Sturnus vulgaris) is not dependent upon the long-day-induced rise in plasma thyroxine. Gen. Comp. Endocrinol. 107, 428–438 10.1006/gcen.1997.6941 (doi:10.1006/gcen.1997.6941) [DOI] [PubMed] [Google Scholar]
- 48.Lessells C. M., Boag P. T. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121 [Google Scholar]
- 49.Gienapp P., Visser M. E. 2006. Possible fitness consequences of experimentally advanced laying dates in Great Tits: differences between populations in different habitats. Funct. Ecol. 20, 180–185 10.1111/j.1365-2435.2006.01079.x (doi:10.1111/j.1365-2435.2006.01079.x) [DOI] [Google Scholar]
- 50.Hegner R. E., Wingfield J. C. 1987. Effects of brood-size manipulations on parental investment, breeding success, and reproductive endocrinology of house sparrows. Auk 104, 470–480 10.1111/j.1420-9101.2005.01034.x (doi:10.1111/j.1420-9101.2005.01034.x) [DOI] [Google Scholar]
- 51.Sharp P. J., Sreekumar K. P. 2001. Photoperiodic control of prolactin secretion. In Avian endocrinology (eds Dawson A., Chaturved C. M.), pp. 245–255 New Delhi, India: Narosa Publishing House [Google Scholar]
- 52.Breitenbach R. P., Nagra C. L., Meyer R. K. 1965. Studies of incubation and broody behaviour in the pheasant (Phasianus colchicus). Anim. Behav. 13, 143–148 10.1016/0003-3472(65)90084-9 (doi:10.1016/0003-3472(65)90084-9) [DOI] [Google Scholar]
- 53.Sockman K. W., Schwabl H., Sharp P. J. 2000. The role of prolactin in the regulation of clutch size and onset of incubation behavior in the American Kestrel. Horm. Behav. 38, 168–176 10.1006/hbeh.2000.1616 (doi:10.1006/hbeh.2000.1616) [DOI] [PubMed] [Google Scholar]
- 54.O'Dwyer T. W., Buttemer W. A., Priddel D. M., Downing J. A. 2006. Prolactin, body condition and the cost of good parenting: an interyear study in a long-lived seabird, Gould's Petrel (Pterodroma leucoptera). Funct. Ecol. 20, 806–811 10.1111/j.1365-2435.2006.01168.x (doi:10.1111/j.1365-2435.2006.01168.x) [DOI] [Google Scholar]
- 55.Wilson A. J., Nussey D. H. 2009. What is individual quality? An evolutionary perspective. Trends Ecol. Evol. 25, 207–214 10.1016/j.tree.2009.10.002 (doi:10.1016/j.tree.2009.10.002) [DOI] [PubMed] [Google Scholar]
- 56.Landys M. M., Ramenofsky M., Wingfield J. C. 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 148, 132–149 10.1016/j.ygcen.2006.02.013 (doi:10.1016/j.ygcen.2006.02.013) [DOI] [PubMed] [Google Scholar]
- 57.Romero L. M. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24 10.1016/S0016-6480(02)00064-3 (doi:10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- 58.Hau M., Ricklefs R. E., Wikelski M., Lee K. A., Brawn J. D. 2010. Cortiocosterone, testosterone, and life-history strategies of birds. Proc. R. Soc. B 277, 3203–3212 10.1098/rspb.2010.0673 (doi:10.1098/rspb.2010.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero L. M. 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255 10.1016/j.tree.2004.03.008 (doi:10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- 60.Dawson A., Goldsmith A. R. 1982. Prolactin and gonadotrophin secretion in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to nesting, incubation, and rearing young. Gen. Comp. Endocrinol. 48, 213–221 10.1016/0016-6480(82)90019-3 (doi:10.1016/0016-6480(82)90019-3) [DOI] [PubMed] [Google Scholar]
- 61.Wingfield J. C., Goldsmith A. R. 1990. Plasma levels of prolactin and gonadal steroids in relation to multiple-brooding and renesting in free-living populations of the song sparrow Melospiza melodia. Horm. Behav. 24, 89–103 10.1016/0018-506X(90)90029-W (doi:10.1016/0018-506X(90)90029-W) [DOI] [PubMed] [Google Scholar]
- 62.Moore I. T., Bonier F., Wingfield J. C. 2005. Reproductive asynchrony and population divergence between two tropical bird populations. Behav. Ecol. 16, 755–762 10.1093/beheco/ari049 (doi:10.1093/beheco/ari049) [DOI] [Google Scholar]
- 63.Silverin B. 1986. Corticosterone-binding proteins and behavioral effects of high plasma levels of corticosterone during the breeding period in the pied flycatcher. Gen. Comp. Endocrinol. 64, 67–74 10.1016/0016-6480(86)90029-8 (doi:10.1016/0016-6480(86)90029-8) [DOI] [PubMed] [Google Scholar]
- 64.Wingfield J. C., Oreilly K. M., Astheimer L. B. 1995. Modulation of the adrenocortical responses to acute stress in arctic birds—a possible ecological basis. Am. Zool. 35, 285–294 10.1093/icb/35.3.285 (doi:10.1093/icb/35.3.285) [DOI] [Google Scholar]
- 65.Romero L. M., Dickens M. J., Cyr N. E. 2009. The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm. Behav. 55, 375–389 10.1016/j.yhbeh.2008.12.009 (doi:10.1016/j.yhbeh.2008.12.009) [DOI] [PubMed] [Google Scholar]
- 66.McGlothlin J. W., Ketterson E. D. 2008. Hormone-mediated suites as adaptations and evolutionary constraints. Phil. Trans. R. Soc. B 363, 1611–1620 10.1098/rstb.2007.0002 (doi:10.1098/rstb.2007.0002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans M. R., Roberts M. L., Buchanan K. L., Goldsmith A. R. 2006. Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. J. Evol. Biol. 19, 343–352 10.1111/j.1420-9101.2005.01034.x (doi:10.1111/j.1420-9101.2005.01034.x) [DOI] [PubMed] [Google Scholar]
- 68.Satterlee D. G., Johnson W. A. 1988. Selection of Japanese quail for contrasting blood corticosterone response to immobilization. Poultry Sci. 67, 25–32 [DOI] [PubMed] [Google Scholar]
- 69.Nussey D. H., Wilson A. J., Brommer J. E. 2007. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 20, 831–844 10.1111/j.1420-9101.2007.01300.x (doi:10.1111/j.1420-9101.2007.01300.x) [DOI] [PubMed] [Google Scholar]
- 70.Visser M. E., van Noordwijk A. J., Tinbergen J. M., Lessells C. M. 1998. Warmer springs lead to mistimed reproduction in Great Tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870 10.1098/rspb.1998.0514 (doi:10.1098/rspb.1998.0514) [DOI] [Google Scholar]
- 71.Whitekiller R. R., Westneat D. F., Schwagmeyer P. L., Mock D. W. 2000. Badge size and extra-pair fertilizations in the House Sparrow. Condor 102, 342–348 10.1650/0010-5422(2000)102[0342:BSAEPF]2.0.CO;2 (doi:10.1650/0010-5422(2000)102[0342:BSAEPF]2.0.CO;2) [DOI] [Google Scholar]