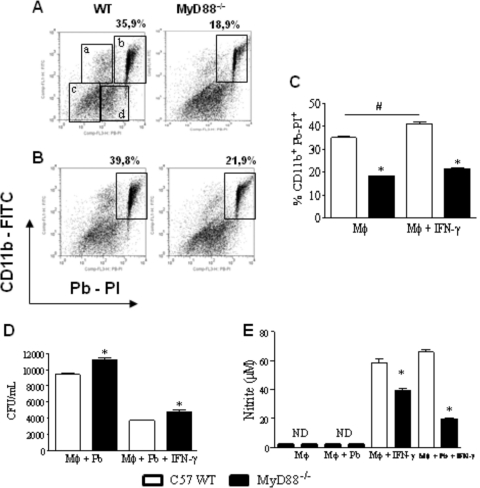
Fig. 1.
Macrophages from MyD88−/− mice have a decreased ability to interact with P. brasiliensis yeast cells. For phagocytic assays, unprimed (A, C) and IFN-γ-primed (20 ng/ml, overnight) (B, C) peritoneal macrophages from MyD88−/− and WT C57BL/6 mice were infected with 1 × 106/well heat-killed PI-labeled yeast cells (1:1 fungus/macrophage ratio). After 2 h, the supernatants were aspirated, the cells washed, and harvested macrophages labeled with FITC anti-CD11b antibodies and analyzed by flow cytometry. Four different cell populations were identified (regions a to d). (a) Single positive FITC-labeled cells (CD11b+ cells that do not phagocytose or adhere to Pb-PI); (b) double-positive cells (PI-Pb-FITC macrophages); (c) dead cells; and (d) nonphagocytosed PI-Pb. For fungicidal assays, IFN-γ-primed and unprimed peritoneal macrophages were infected with P. brasiliensis yeast cells at a macrophage/yeast ratio of 25:1. After 48 h of cocultivation, the supernatants were obtained to characterize NO and cytokine production; monolayers were lysed and assayed for the presence of viable yeast cells by a CFU assay (D). Supernatants were used to determine the levels of nitrites using the Griess reagent (E). Data are means ± standard errors of the means (SEM) of results from quintuplicate samples from one experiment representative of three independent determinations. # and *, P < 0.05.