Abstract
Background:
The glycemic penalty index (GPI) is a measure to assess blood glucose (BG) control in critically ill adult patients but needs to be adapted for children and infants.
Method:
The squared differences between a clinical expertise penalty function and the corresponding polynomial function are minimized for optimization purposes. The average of all penalties (individually assigned to all BG readings) represents the patient-specific GPI.
Results:
Penalization in the hypoglycemic range is more severe than in the hyperglycemic range as the developing brains of infants and children may be more vulnerable to hypoglycemia. Similarly, hypoglycemia is also more heavily penalized in infants than in children.
Conclusions:
Extending the adult GPI toward the age-specific GPI is an important methodological step. Long-term clinical studies are needed to determine the clinically acceptable GPI cut-off level.
Keywords: blood glucose, children, critically ill patients, evaluation, glycemic penalty index, infants
Introduction
Critically ill patients have a severe dysregulation of their glucose homeostasis. Observational studies have shown that hyperglycemia as well as hypoglycemia are associated by a J-curve relation, with increased risk of death in critically ill patients.1 Seminal single-center (Leuven) studies have shown that strictly normalizing blood glucose (BG) levels [aiming at tight glycemic control (TGC)] by intensive insulin therapy (IIT) decreases the risk of death.2–4
In the wake of the Leuven studies, large, multicenter, follow-up trials were done.5–7 Due to methodological disparity in the execution of the complex intervention of TGC,8,9 these studies either could not confirm this survival benefit or even resulted in an increased mortality in patients who were treated with IIT. It is now generally accepted that the methodological aspects of TGC have been underestimated, possibly resulting in larger than expected swings in BG levels.
To assess these BG swings and hence quantify the efficacy of TGC, variables such as the hyperglycemic index have been suggested.10 Weaknesses related to this index (e.g., linear relationship assumptions between measurements, sensitivity to outlier measurements) led to the development of the glycemic penalty index (GPI).11 This index encompasses the overall BG dynamic behavior in a single number for each adult patient and has the following advantages: hypo- and hyperglycemic deviations do not compensate for each other (as is the case of average BG); BG outliers do not shift the evaluation (as is the case of average BG, hyperglycemic index); and only the measured BG values are taken into account (there is no assumed relationship between BG measurements). Moreover, it has been shown that low GPIs were associated with reduced mortality for critically ill adult patients.12
Aiming for lower targets in infants (0–1 year: 50–80 mg/dl) and children (1–16 years: 70–100 mg/dl) and the associated higher risk of hypoglycemia makes TGC even more difficult than in adults.4 Despite the elevated incidence of hypoglycemia, short-term outcomes in critically ill infants and children were improved and neurological injury biomarkers did not detect neurological damage.13 The long-term neurological outcome of these patients is being investigated. Not only hyperglycemia and the number and severity of hypoglycemic episodes but also glycemic variability may possibly affect this long-term outcome, The adult GPI therefore needs to be extended to a GPI for children and infants.
Methods
Glycemic threshold values per age group were obtained from the literature (Table 1).4,11,14–17 The target BG range of the first (and only finished) randomized controlled trial on IIT in pediatric critically ill patients served as the normoglycemic range because of its associated important clinical benefits.4
Table 1.
Overview of the Different Penalties: BG Ranges (in mg/dl) for Adults,11 Children, and Infants
| Range Number | Clinical condition | Adults | Children | Infants | Penalty | Reference |
|---|---|---|---|---|---|---|
| 1 | Hypoglycemic alarm | BG < 40 | BG < 40 | BG < 40 | 3 | 14–15 |
| 2 | Hypoglycemia | 40 ≤ BG < 60 | 40 ≤ BG < 60 | / | 2 | 14–16 |
| 3 | Slight hypoglycemia | 60 ≤ BG < 80 | 60 ≤ BG < 70 | 40 ≤ BG < 50 | 1 | 15–16 |
| 4 | Normoglycemic range | 80 ≤ BG ≤ 110 | 70 ≤ BG ≤ 100 | 50 ≤ BG ≤ 80 | 0 | 4 |
| 5 | Slight hyperglycemia | 110 < BG ≤ 150 | 100 < BG ≤ 125 | 80 < BG ≤ 125 | 1 | 17 |
| 6 | Hyperglycemia | 150 < BG ≤ 200 | 125 < BG ≤ 150 | 125 < BG ≤ 150 | 2 | 16–17 |
| 7 | Hyperglycemic alarm | 200 < BG | 150 < BG ≤ 200 | 150 < BG ≤ 200 | 3 | 16–17 |
| 8 | Hyperglycemic alarm (high) | / | 200 < BG | 200 < BG | 4 | 4,16 |
Each BG range corresponded to a penalty, resulting in a staircase “clinical expert” penalty function. Higher penalties were appointed to larger deviations from the normoglycemic range (with penalty 0). The staircase function was transformed into a smooth polynomial function for both hypo- and hyperglycemia to avoid abrupt changes. Next, this smooth polynomial function was optimized, considering age-dependent BG normoglycemic target ranges (see Table 1), hypo- and hyperglycemic alarm levels (40 and 200 mg/dl), and minimum and maximum BG levels (20 and 250 mg/dl) to avoid misinterpretations caused by BG outliers.
Hence, the hypoglycemic range for children and infants in the optimization process was limited to 20–69 and 20–49 mg/dl, whereas the hyperglycemic range was set at 101–250 and 81–250 mg/dl, respectively. In the optimization process, the squared differences between the staircase clinical expert function and the polynomial function were minimized using least squares and the Nelder-Mead algorithm.11,18–19
Results
The optimized polynomial function used to compute the penalty index for each BG measurement can be mathematically formulated as follows:
For critically ill child patients and for time step t = 1 to Ntotal,
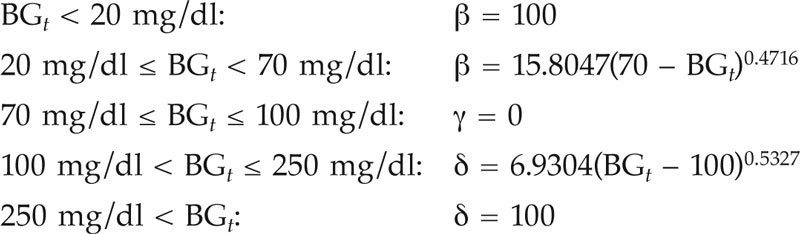
For critically ill infant patients and for time step t = 1 to Ntotal,
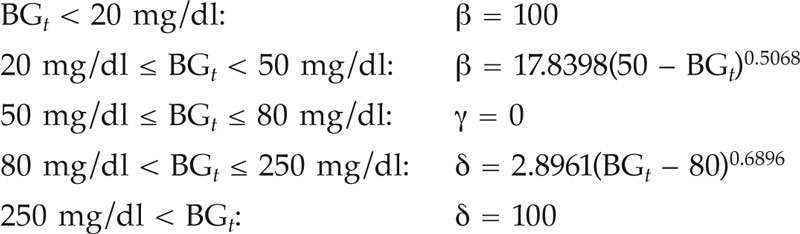
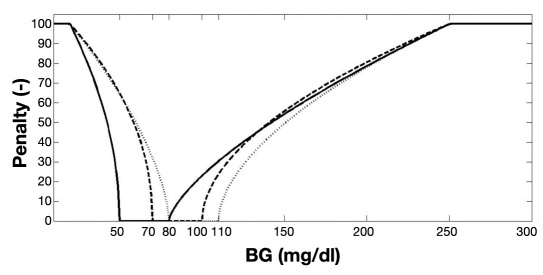
where β, γ, and δ are the penalties for the hypoglycemic, normoglycemic, and hyperglycemic ranges, respectively. The smoothed penalty functions are illustrated in Figure 1.
Figure 1.
The age-dependent staircase “clinical expert” penalty function (see Table 1) is here transformed into an optimized smooth penalty function for adults (dotted line, see also Van Herpe and colleagues11), children (dashed line), and infants (solid line).
The GPI per patient is computed as follows:
| (1) |
where Nhypo and Nhyper symbolize the number of BG measurements in the hypo- and hyperglycemic range. The total number of BG measurements available for that patient is represented as Ntotal. The computation of GPI, to be performed for each patient, returns a number between 0 (all BG measurements in normoglycemic range) and 100 (all BG measurements above 250 mg/dl or below 20 mg/dl).
Discussion
Aiming to assess the effect of TGC on glucose variability in critically ill pediatric patients, we transformed the adult GPI to a child and infant GPI based on age-dependent normal BG ranges as set in the randomized controlled trial.4 The obtained GPI formula generates a number between 0 and 100 with an ideal level of 0 (indicating that all measured BG values fall within the normoglycemic range).
The optimized smooth penalty function reflects the J-shaped relationship between BG level and mortality risk. This penalty function shows a steep behavior in the hypoglycemic range, while it evolves more gradually in the hyperglycemic range. Indeed, relatively small absolute deviations in the hypoglycemic range are potentially more dangerous than similar deviations in the hyperglycemic range. This explains the steeper penalty curve in the hypoglycemic range. Therefore, the hypoglycemic penalty scores [β = 15.8047(70 – BGt)0.4716 for children and β = 17.8398(50 – BGt)0.5068 for infants] are higher than the hyperglycemic penalty scores [δ = 6.9304(BGt – 100)0.5327 for children and δ = 2.8961(BGt – 80)0.6896 for infants] for absolute deviations from normoglycemia.
The developing brain of infants and children may also be more vulnerable to hypoglycemia. Accordingly, the hypoglycemic penalty score of β = 7.4680(80 – BGt)0.6337 for the adult population11 was much lower than in the pediatric ICU population for absolute deviations from normoglycemia (see also Figure 1). Also the hypoglycemic penalty score in the extended GPI was higher in infants than in children.
The proposed infant and child GPI methodology is mainly founded on the BG target ranges that were set in the randomized controlled trial of IIT in the critically ill pediatric population.4 These ranges were found to have favorable effects on mortality and morbidity. The use of a GPI cut-off level would facilitate the clinical use of this rather theoretical GPI methodology. This cut-off level would indicate the clinical acceptability of BG control performance by taking into account BG measurement errors caused by sensor inaccuracies (due to the BG measurement device) and methodology inaccuracies (due to sample handling) as was earlier realized for the adult GPI.11–12
Further studies are needed to fine tune this GPI cut-off level and to confirm probable associations between GPIs below this cut-off level and reduced morbidity and mortality. The development of this age-specific GPI methodology, however, is an important first step as BG control in critically ill child and infant patients is substantially different from BG control in critically ill adult patients and should be specifically evaluated, accordingly.
Conclusions
The GPI summarizes the BG dynamic behavior of critically ill patients in a single (patient-specific) number by averaging the penalties that were assigned to all BG measurements of that patient. The extended (age-specific) GPI has taken into account the increased risk of hypoglycemia in critically ill children and infants. Further long-term studies are needed to fine tune the GPI cut-off level that indicates the clinical acceptability of BG control performance and to show probable associations between lower GPI levels and improved clinical outcomes.
Abbreviations
- (BG)
blood glucose
- (GPI)
glycemic penalty index
- (ICU)
intensive care unit
- (IIT)
intensive insulin therapy
- (TGC)
tight glycemic control
References
- 1.Bagshaw SM, Egi M, George C, Bellomo R. Early blood glucose control and mortality in critically ill patients in Australia. Crit Care Med. 2009;37(2):763–770. doi: 10.1097/CCM.0b013e318194b097. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 3.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters P, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 4.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373(9663):547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 5.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 6.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 7.Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berghe G, Bouillon R, Mesotten D. Glucose control in critically ill patientsy. N Engl J Med. 2009;361(1):89. doi: 10.1056/NEJMc090812. author reply 91-2. [DOI] [PubMed] [Google Scholar]
- 9.Van den Berghe G, Schetz M, Vlasselaers D, Hermans G, Wilmer A, Bouillon R, Mesotten D. Clinical review: intensive insulin therapy in critically ill patients: NICE-SUGAR or Leuven blood glucose target? J Clin Endocrinol Metab. 2009;94(9):3163–3170. doi: 10.1210/jc.2009-0663. [DOI] [PubMed] [Google Scholar]
- 10.Vogelzang M, van der Horst I, Nijsten M. Hyperglycaemic index as a tool to assess glucose control: a retrospective study. Crit Care. 2004;8(3):R122–R127. doi: 10.1186/cc2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Herpe T, De Brabanter J, Beullens M, De Moor B, Van den Berghe G. Glycemic penalty index for adequately assessing and comparing different blood glucose control algorithms. Crit Care. 2008;12(1):R24. doi: 10.1186/cc6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, Van den Berghe G. Dynamic characteristics of blood glucose time series during the course of critical illness: effects of intensive insulin therapy and relative association with mortality. Crit Care Med. 2010;38(4):1021–1029. doi: 10.1097/CCM.0b013e3181cf710e. [DOI] [PubMed] [Google Scholar]
- 13.Vanhorebeek I, Gielen M, Boussemaere M, Wouters PJ, Grandas FG, Mesotten D, Van den Berghe G. Glucose dysregulation and neurological injury biomarkers in critically ill children. J Clin Endocrinol Metab. 2010;95(10):4669–4679. doi: 10.1210/jc.2010-0805. [DOI] [PubMed] [Google Scholar]
- 14.Zijlmans WC, Van Kempen AA, Serlie MJ, Sauerwein HP. Glucose metabolism in children: influence of age, fasting, and infectious diseases. Metabolism. 2009;58(9):1356–1365. doi: 10.1016/j.metabol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Preissig CM, Rigby MR. A disparity between physician attitudes and practice regarding hyperglycemia in pediatric intensive care units in the United States: a survey on actual practice habits. Crit Care. 2010;14(1):R11. doi: 10.1186/cc8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118(1):173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Assocation of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5(4):329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 18.Nelder JA, Mead R. A simplex method for function minimization. Comput J. 1965;7(4):308–313. [Google Scholar]
- 19.Maddala G. 3rd ed. Chichester (UK): Wiley; 2001. Introduction to econometrics. [Google Scholar]