Abstract
The recent advances in molecular biology have revolutionized all aspects of dentistry. DNA, the language of life yields information beyond our imagination, both in health or disease. DNA fingerprinting is a tool used to unravel all the mysteries associated with the oral cavity and its manifestations during diseased conditions. It is being increasingly used in analyzing various scenarios related to forensic science. The technical advances in molecular biology have propelled the analysis of the DNA into routine usage in crime laboratories for rapid and early diagnosis. DNA is an excellent means for identification of unidentified human remains. As dental pulp is surrounded by dentin and enamel, which forms dental armor, it offers the best source of DNA for reliable genetic type in forensic science. This paper summarizes the recent literature on use of this technique in identification of unidentified human remains.
Keywords: DNA analysis, DNA profiling, forensic odontology
Introduction
The realization that DNA lies behind all the cell's activities led to the development of molecular biology. The development of the methods and techniques to study processes at the molecular level has led to new and powerful ways of isolating, analyzing, manipulating and exploiting nucleic acids. DNA fingerprinting is the result of such an endeavor.
Forensic dental identification is at technological cross roads. The role of dental restorations, prosthesis and radiological identification as the main stray of forensic odontology has declined lately, whereas molecular biology and laboratory procedures are rapidly increasing in efficiency and availability.[1] The tooth is the most valuable source to extract DNA since it is a sealed box preserving DNA from extreme environmental conditions, except its apical entrance. This has prompted the investigation of various human tissues as potential source of genetic evidentiary material. Recently teeth have been the subject of DNA studies as the dental hard tissue physically encloses the pulp and offers an anatomical configuration of great durability.[2] Moreover, when morphologically evaluated, even a single tooth provides valuable information regarding the individual to whom the tooth belongs.[3–5]
Historical Review
Jeffery (1985)[6] described hypervariable regions of human DNA using multilocus probes and the applicability of these DNA polymorphisms to the individualization of human blood and tissues. The potential forensic applications of DNA analysis in resolving disputed parentage problems, identification of human remains as well as in the individualization of blood and body fluids in crime laboratories were immediately recognized.[7]
Polymerase chain reaction (PCR), originally introduced by Saiki et al.[8] and subsequently automated by Mullis and Faloona,[9] has emerged as a powerful tool in forensics for the exponential in vitro amplification of specific sequences of interest from minute quantities of DNA or RNA and was rapidly applied to forensic odontology.
Schwartz et al. in 1991 isolated high molecular weight (HMW) from the teeth under different environmental conditions such as varying pH, humidity, temperature, storage, etc. It was determined that the environmental conditions examined did not affect the ability to obtain HMW human DNA from dental pulp.[10]
Pötsch et al. in 1992 performed genomic dot blot hybridization for sex determination using the biotinylated repetitive DNA probe pHY 2.1 and sex was correctly classified in all cases using 50-100 ng target DNA from pulp.[11]
Among the several cases described in the literature with DNA isolation from teeth, a very important report was published by Sweet and Sweet (1995).[12] This paper presents a case of human remains identification, by a preserved unerupted third molar which enabled 1.35 μg DNA extraction from the dental pulp.
Dental identification
This is the most common role of the forensic odontologist. Dental identification of a body may sometimes be necessary due to the circumstances of the death. It must be noted that the vast majority of deceased are identified by non-dental means, namely visual identification by a family member and fingerprint identification. But when neither of these are possible due to disfiguration or decomposition, that may render the deceased unrecognizable, or where people are not available to make a positive visual identification, then dental identification may be required. Confirming the identity of the deceased is not only important for the family and friends of the deceased from an emotional and grieving aspect but is also a legal requirement.[2]
Different techniques of identifying individual through dental means are available. Currently there are four types of personal identification circumstances that use teeth, jaw and orofacial characteristics, which include comparative dental identification, reconstructive postmortem, dental profiling and DNA profiling.[13] The most commonly used technology of comparative dental identifications has several disadvantages [Table 1].[13,14]
Table 1.
Disadvantages of comparative dental identification

These voids form the basis for progression in field of DNA identification. DNA technology made molecular analysis of ancient samples possible.
Basis for DNA fingerprinting
DNA fingerprinting or DNA profile are encrypted sets of numbers that reflect a persons DNA makeup, which can also be used as the persons identifier.[15] Gene is a segment of DNA that codes for a particular protein. This accounts for only 2-5% of entire cellular DNA. The function of the remaining 95% or more of the DNA is not known and is called as non-coding DNA or junk DNA. The non-coding DNA generally may either be as single copy acting as a spacer DNA between coping regions of genome or exist in multiple copies this is being called repetitive DNA (20-30%). The repetitive sequence is highly polymorphic and unique to each individual. It appears as long tandem repeats (midi satellites), short tandem repeats (STR; mini satellites) and interspersed repetitive sequences [Figure 1]. The extreme variability in pattern of mini satellites detected by probe together with stable inheritance in usual Mendelian manner of individual pattern forms the basis of DNA fingerprinting. Variations in DNA sequence called polymorphisms can be used both to differentiate and to correlate individuals.[16]
Figure 1.
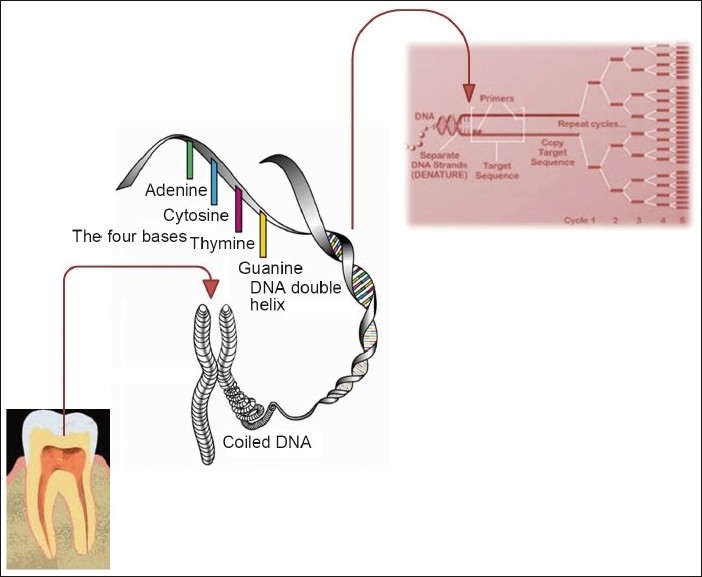
Schematic photograph showing replication of DNA by PCR
Anatomical location for DNA in tooth
The teeth differ in form and size but have similar histological structure. The dentin is a connective tissue that forms the major structural axis of the tooth and is hardly exposed to the oral environment. The dentin on the crown of the tooth is covered by enamel. The enamel has an ectodermic origin and is an extremely mineralized tissue. Furthermore, it is an acellular and avascular structure without nerves. The root dentin is covered by the cement, another type of calcified connective tissue. Soft tissue within coronal and radicular pulp chamber consists of odontoblasts, fibroblasts, endothelial cells, peripheral nerve, undifferentiated mesenchymal cells and nucleated components of blood which are rich sources of DNA. Other less frequently used anatomical locations of DNA includes, odontoblastic process that extend into dentinal tubules, soft tissue within accessory canals, cellular cementum, adherent bone and periodontal ligament fibres.[4]
Stabilization of DNA in a tooth
Extraction of DNA from the human body remains a difficult task and depends upon numerous environmental factors and extraction procedures. Experience has shown that DNA from hard tissues like bone and teeth are most stable even after putrefaction of bodies.[17]
Schwartz (1991)[10] isolated HMW DNA from teeth at 4°C upto 6 weeks. At 25°C, HMW DNA can be isolated after 19 years. At 37°C, teeth can yield HMW DNA following storage for 6 months. TC Boles (1995)[17] could successfully extract DNA from teeth that had been buried up to 80 years. It is possible to discriminate one individual from all others with a high level of confidence by starting with only 1 ng or less of target DNA whereas, the amount of DNA that can be recovered from molar teeth with pulp volume of 0.023-0.031cc is nearly 15-20 mg.
In a study conducted by Pötsch, et al. (1992),[11] the total production of genomic DNA obtained from a dental sample ranged from 6 to 50 μg DNA. The results were obtained from DNA extracted from the dental pulp and did not show any difference when compared to the patterns obtained from DNA isolated from blood samples or available lung tissues.
Remualdo (2004)[18] evaluated the PCR amplification of DNA retrieved from teeth subjected to heat (200°C, 400°C, 500°C and 600°C) during 60 minutes, testing three different extraction methods (organic; ammonia acetate/isopropanol and silica). Using the organic method for genomic DNA extraction, 50% of samples subjected to burning were amplified, but only at lower temperatures (200°C and 400°C). At higher temperatures (500°C and 600°C), the isopropanol/ammonia acetate extraction method yielded better results, mainly for extraction of mitochondrial DNA (mtDNA).
A recent study has found out that mtDNA can be sourced from dentine powder obtained via cryogenic grinding, and also via dentine in the case of root-filled tooth.[19]
The factors affecting the availability of target DNA in a tooth depends upon different factors [Table 2].
Table 2.
Variables affecting amount of DNA in a tooth[10]

The pulp produced the strongest PCR amplification signals, while dentin and cementum signals were very similar to each other.[20]
A preserved unerupted third molar enabled DNA extraction from the dental pulp (1.35 μg), which was an excellent source of HMW genomic DNA.[12]
DNA fingerprinting is a multistep laboratory process which involves removal of pulp from tooth, DNA isolation and analysis of DNA. Different techniques and approaches are available to extract DNA from the teeth [Tables 3 and 4].
Table 3.

Table 4.
Methods of analyzing different types of DNA polymorphisms
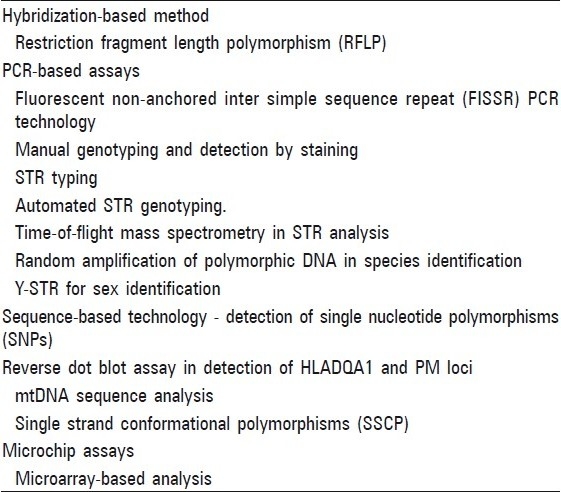
There are numerous methods of isolation of DNA [Table 3], few commonly used in forensic dentistry are discussed below
RFLP methods
Digestion is done by isolating and quantifying DNA, which is chopped into fragments with the help of special enzymes called as restriction endonucleases. These enzymes work like molecular scissors, cleaves the DNA at specific sites, each recognizing a particular sequence. These DNA scissors are specifically chose to cut DNA at sites which are not found within the sequence of “tandem repeats” rather in conserved less variable regions. The cut fragments will contain variable number of tandem repeats (VNTR) of varying lengths, there by producing DNA fragments of various sizes.[11]
The VNTR testing, which may present short repeated sequences of intermediate size [15-65 base pairs (bp)], is rarely used in forensic analyses due to the poor quality DNA provided with this method.
PCR methods
PCR is used to amplify the amount of DNA material available, so that sufficient quantity is available to carry our DNA analysis [Figure 1]. To carry out the reaction special enzyme and DNA primers are required. These primers are like probes with known constant sections of DNA but not labeled. They are designed to known constant sections of DNA at the ends of variable region to be amplified. The principle of PCR is that the DNA is capable of duplicating itself. This is done by unwinding the strands of DNA and each strand acts as a template for synthesis of new strand. By PCR technique we can amplify specific DNA segments dependent on the primer employed. The standard PCR reaction runs through 30 cycles in a couple of hours which results in amplification of original DNA by over 109 times.[14,15]
The DNA found can be genomic (found in the nucleus) and mtDNA (in the mitochondria). The teeth are an excellent source of genomic and mtDNA because PCR analyses allow comparing the collected postmortem samples to known antemortem samples or parental DNA.[19] Main advantage of mtDNA is the high number of copies per cell (from hundreds to thousands of organelles).
STR analysis
In forensic samples, the study of DNA (genomic and mitochondrial) is usually performed by STR analysis, which can be defined as hypervariable regions of DNA that present consecutive repetitions of fragments that have 2-7 bp.[22] The Federal Bureau of Investigation has chosen 13 specific STR loci to serve as the standard for the Combined DNA index system.[23] STR was used on 45 DNA samples from teeth obtained from unidentified bodies buried in 1995 and exhumed in 2000, and pulp showed strongest PCR amplification signals.[20] STR testing is being used for forensic casework, making a revolution on human identification and paternity tests.
mtDNA analysis
mtDNA differs from nuclear DNA in its location, its quantity in the cell, its mode of inheritance and its sequence. mtDNA analysis can be used to examine the DNA from samples that cannot be analyzed by RFLP or STR. mtDNA analysis uses DNA extracted from another cellular organelle called a mitochondrion. While older biological samples that lack nucleated cellular material, such as hair, bones and teeth, cannot be analyzed with STR and RFLP, they can be analyzed with mtDNA. In the investigation of cases that have gone unsolved for many years, mtDNA is extremely valuable. It is better than nuclear genome as it is passed through maternal lineage and has 100-1000 copies of mtDNA genome.[24] This analysis can be used in the tooth especially dentin and cement which contain enough DNA to allow the amplification of the mtDNA, which can be used in the human identification.[20] Silva (2007)[22] stated that the analysis of mtDNA for forensic purposes is restricted to ancient tissues, such as bones, hair and teeth, in which the nuclear DNA cannot be analyzed.
Y-chromosome analysis
The Y-chromosome is passed directly from father to son, so analysis of genetic markers on the Y-chromosome is especially useful for tracing relationships among males or for analyzing biological evidence involving multiple male contributors. Since the beginning of the 90s the field of forensic Y-chromosome analysis has been successfully developed to become common place in laboratories working in crime casework all over the world. In Y-STR analysis, specific regions of DNA on the Y-male chromosome are targeted and copied many times. Y-STR DNA profiling system selectively targets male DNA even in the presence of large amounts of female DNA. The Forensic Science Service, led by Dr. Gill, developed and implemented Low Copy Number DNA profiling in late 1990.[25] ESR Principal Scientist, Dr. John Buckleton, worked with Dr. Gill and others at the UK Forensic Science Service to establish the technique and develop interpretation guidelines.[26] Determination of sexes of all freshly collected samples within 24 hours and after 1 month of extraction, respectively, gave 100% result. However, PCR was not found to be an effective method for sex determination after 6 months post-extraction.[27]
AmpFLP
Another technique, AmpFLP, or amplified fragment length polymorphism was also put into practice during the early 1990s.[28] This technique was also faster than RFLP analysis and used PCR to amplify DNA samples. AmpFLP analysis can be highly automated, and allows for easy creation of phylogenetic trees based on comparing individual samples of DNA. Owing to its relatively low cost and ease of set-up and operation, AmpFLP remains popular in lower income countries.[29]
SNP
SNP detection technologies are used to scan for new polymorphisms and to determine the allele(s) of a known polymorphism in target sequences.[30] SNP detection technologies have evolved from labor intensive, time consuming, and expensive processes to some of the most highly automated, efficient, and relatively inexpensive methods. Local, target, SNP discovery relies mostly on direct DNA sequencing or on denaturing high performance liquid chromatography.[31] The demand for SNP genotyping is great; however, no one method is able to meet the needs of all studies using SNPs. Despite the considerable gains over the last decade, new approaches must be developed to lower the cost and increase the speed of SNP detection.
Application of DNA fingerprinting in forensic odontology include instances were DNA is not available in any other part of the body as in case of major disasters like plane crash, charred bodies, decomposed bodies and building collapse.[2,4–6] Few interesting cases reported in the literature is mentioned below.
A very interesting case was presented by Sweet and Sweet (1995) in which a victim was incinerated and her body was completely carbonized and DNA extraction by usual method was not possible. However, her body was identified after an unerupted third molar enabled extraction of DNA.[12]
The Indian Ocean tsunami of 26 December 2004 created major challenges for forensic identification of dead bodies. DNA profiling were useful in identification of those bodies where other dental methods have been unsuccessful.[32]
Egypt's most powerful female ruler Queen Hatshepsut mummy identity had been in question for many years. DNA tests were carried on molar teeth, which were retrieved from the wooden box with her name inscribed on it. Research Centre in Cairo has promising preliminary results suggesting the identity of the queen.[33]
Conclusions
The arrival of DNA finger printing has revolutionized the concept of identification. It is reasonable to anticipate that future advances in DNA technology will reduce the time and cost factor for identification of unknown deceased. Meanwhile clinical observation of available medical and dental patient records remains the gold standard for forensic pathology.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Pakhmode VK, Pakhmode CK. Dental DNA for genetic finger printing. IAOFG. 1998;1:4–5. [Google Scholar]
- 2.Sweet D. Why a dentist for identification? Dent Clin North Am. 2001;45:237–51. [PubMed] [Google Scholar]
- 3.Smith BC, Fisher DL, Weedn VW, Warnock GR, Holland MM. A systemic approach to the sampling of dental DNA. J Forensic Sci. 1993;38:1194–209. [PubMed] [Google Scholar]
- 4.Sweet D, Hildebrand D, Phillips D. Identification of skeleton using DNA from teeth and a PAP smear. J Forensic Sci. 1999;44:630–3. [PubMed] [Google Scholar]
- 5.Sweet D, Hildebrand D. Recovery of DNA from human teeth by cryogenic grinding. J Forensic Sci. 1998;43:1199–202. [PubMed] [Google Scholar]
- 6.Jeffreys AJ, Wilson V, Thein SL. Hypervariable ‘minisatellite’ regions in human DNA. Nature. 1985;314:67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- 7.Dodd BE. DNA finger printing in matters of family and crime. Nature. 1985;318:506–7. doi: 10.1038/318506a0. [DOI] [PubMed] [Google Scholar]
- 8.Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, et al. Enzymatic amplification of ss-globin sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–4. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 9.Mullis KB, Faloona FA. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–50. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz TR, Schwartz EA, Mieszerski L, McNally L, Kobilinsky L. Characterization of DNA obtained from teeth subjected to various environmental conditions. J Forensic Sci. 1991;36:979–90. [PubMed] [Google Scholar]
- 11.Pötsch L, Meyer U, Rothschild S, Schneider PM, Rittner C. Application of DNA techniques for identification using human dental pulp as a source of DNA. Int J Legal Med. 1992;105:139–43. doi: 10.1007/BF01625165. [DOI] [PubMed] [Google Scholar]
- 12.Sweet DJ, Sweet CH. DNA analysis of dental pulp to link incinerated remains of homicide victim to crime scene. J Forensic Sci. 1995;40:310–4. [PubMed] [Google Scholar]
- 13.Sweet D. Forensic dental identification. Forensic Sci Int. 2010;201:3–4. doi: 10.1016/j.forsciint.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Brannon RB, Kessler HP. Problems in mass disaster dental identification: A retrospective review. J Forensic Sci. 1999;44:123–7. [PubMed] [Google Scholar]
- 15.Wikipedia: the free encyclopedia. [last accessed on 2009 Nov 29]. Available from: http://www.en.wikipedia.org/wiki/DNA_profiling .
- 16.Alonso A, Martin P, Albarrán C, Garcia P, Fernandez de Simon L, Jesús Iturralde M, et al. Challenges of DNA profiling in mass disaster investigations. Croat Med J. 2005;46:540–8. [PubMed] [Google Scholar]
- 17.Boles TC, Snow CC, Stover E. Forensic DNA testing on skeletal remains from mass grave: A pilot project in Guatemala. JFSCA. 1995;45:349–55. [PubMed] [Google Scholar]
- 18.Remualdo VR. Assessment of three methods of extraction of DNA of teeth of humans subjected to heat (dissertation), São Paul SP: Faculty of Dentistry, University of São Paul. 2004 [Google Scholar]
- 19.Shiroma CY, Fielding CG, Lewis JA, Jr, Gleisner MR, Dunn K. A minimally destructive technique for sampling dentin powder for mitochondrial DNA testing. J Forensic Sci. 2004;49:791–5. [PubMed] [Google Scholar]
- 20.Malaver PC, Yunis JJ. Different dental tissues as source of DNA for human identification in forensic cases. Croat Med J. 2003;44:306–9. [PubMed] [Google Scholar]
- 21.Alakoc YD, Aka PS. “Orthograde entrance technique” to recover DNA from ancient teeth preserving the physical structure. Forensic Sci Int. 2009;188:96–8. doi: 10.1016/j.forsciint.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 22.da Silva RH, Sales-Peres A, de Oliveira RN, de Oliveira FT, Sales-Peres SH. Use of DNA technology in forensic dentistry. J Appl Oral Sci. 2007;15:156–61. doi: 10.1590/S1678-77572007000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakraborty R, Stivers DN, Su B, Zhong Y, Budowle B. The utility of short tandem repeat loci beyond human identification: Implications for development of new DNA typing systems. Electrophoresis. 1999;20:1682–96. doi: 10.1002/(SICI)1522-2683(19990101)20:8<1682::AID-ELPS1682>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 24.Holland MM, Fisher DL, Mitchell LG, Rodriquez WC, Canik JJ, Merril CR, et al. Mitochondrial DNA sequence analysis of human skeletal remains: Identification of humans from Vietnam War. J Forensic Sci. 1993;38:542–53. [PubMed] [Google Scholar]
- 25.Gill P. Application of low copy number DNA profiling. Croat Med J. 2001;42:229–32. [PubMed] [Google Scholar]
- 26.Gill P, Sparkes R, Pinchin R, Clayton T, Whitaker J, Buckleton J. Interpreting simple STR mixtures using allele peak areas. Forensic Sci Int. 1998;91:41–53. doi: 10.1016/s0379-0738(97)00174-6. [DOI] [PubMed] [Google Scholar]
- 27.Lucotte G, Mercier G. Brief communication: Y-chromosome haplotypes in Egypt. Am J Phys Anthropol. 2003;121:63–6. doi: 10.1002/ajpa.10190. [DOI] [PubMed] [Google Scholar]
- 28.Hochmeister MN, Budowle B, Borer UV, Eggmann U, Comey CT, Dirnhofer R. Typing of DNA extracted from compact bone from human remains. J Forensic Sci. 1991;36:1649–61. [PubMed] [Google Scholar]
- 29.Comey CT, Koons BW, Presley KW, Smerick JB, Sobieralski CA, Stanley DM, et al. Extraction strategies for amplified fragment length polymorphism analysis. JFSCA. 1994;39:1254–69. [Google Scholar]
- 30.Budowle B. SNP typing strategies. Forensic Sci Int. 2004;146:S139–42. doi: 10.1016/j.forsciint.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 31.Kwok PY, Chen X. Detection of single nucleotide polymorphisms. Curr Issues Mol Biol. 2003;5:43–60. [PubMed] [Google Scholar]
- 32.Hirsch C, Brondolo T, Butcher B. New York: city of New York office of chief medical examiner; 2005. Report to Dr. Surachai, Minister of Public Health, Thailand, and Dr. William Aldis, WHO representative to Thailand World Health Organization. Assessment of victim identification operations: Thailand tsunami disaster; p. 11. [Google Scholar]
- 33.Angelique C. University helps with Pharaoh's identity. BDJ. 2007;203:123. [Google Scholar]


