Fig. 3. Signaling pathways involved in regulation and modulation of the smooth muscle KATP channel.
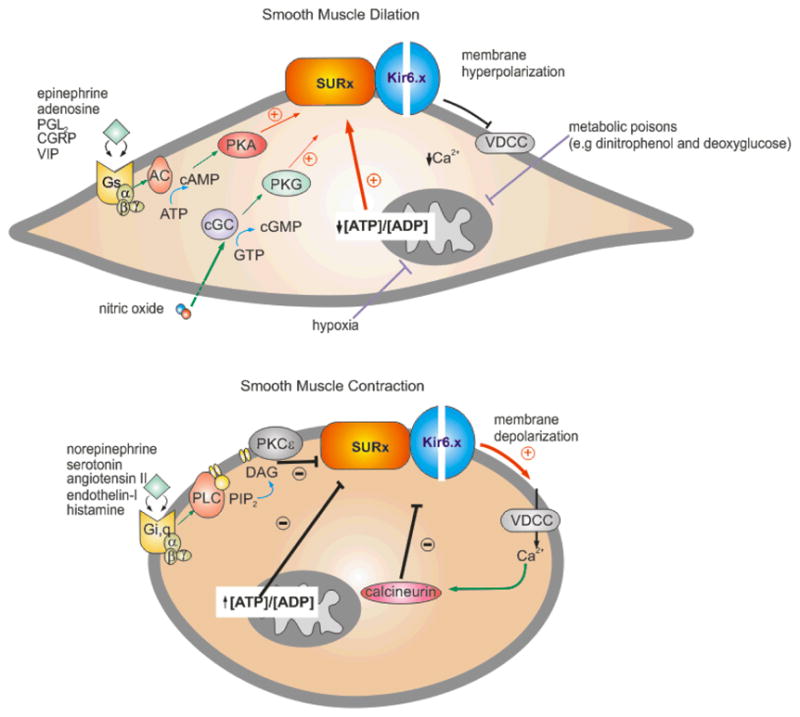
The KATP channel determines, in part, the membrane potential of the smooth muscle cell and, hence, the contractile state. Vasoactive factors that inhibit KATP activity cause membrane depolarization, activation of voltage-dependent Ca2+-channels (VDCC), a rise in intracellular [Ca2+] and smooth muscle contraction. Conversely, factors that activate KATP prevent the depolarization-dependent rise in [Ca2+] and promote smooth muscle dilation. (A) Endogenous vasodilators, including epinephrine, adenosine, prostacyclin (PGl2), calcitonin-generated peptide (CGRP), and the vasoactive intestinal peptide (VIP), stimulate KATP activity through the classic G-protein (Gs)/adenylate cyclase (AC)/protein kinase A (PKA) signaling pathway. Serine/threonine phosphorylation at several key residue in SUR2B (Thr633, Ser1387, and Ser1465) is thought to underlie channel activation. Conversely, nitric oxide activates KATP through the protein kinase G (PKG) signaling pathway. Hypoxia and metabolic poisons (dinitrophenol and deoxygluxose) indirectly activate KATP by suppressing oxidative phosphorylation, resulting in a decrease in the ATP/ADP levels. The K+-channel opener drugs (KCOs) (eg. pinacidil and diazoxide) promote their anti-hypertensive effects by opening vascular smooth KATP channels via interaction with the SUR2B subunit. (B) Vasoconstrictor agonists that target VSM KATP to promote contraction include the neurotransmitter serotonin (5-HT), angiotensin-II, endothelin-I, and the pro-inflammatory histamine. Binding of vasoconstrictors to the G-protein receptor subtypes, Gi or Gq, indirectly activates the PKC-ε isoform. In turn, PKCε-mediated phosphorylation of a serine-rich motif in the C-terminus of Kir6.1 results in a decrease in the frequency of channel openings. Ca2+-activated calcineurin (phosphatase type 2B) inhibits VSM KATP although the mechanism is unclear. Sulfonylurea compounds (e.g glibenclamide) induce vasoconstriction by binding to the SUR2B subunit causing KATP channel closure.
