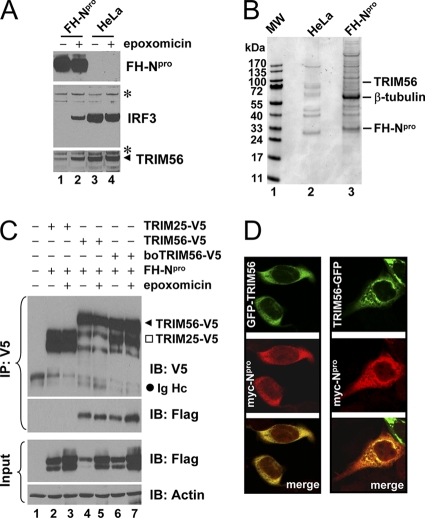
Fig. 1.
Identification of TRIM56 as a protein interaction partner of BVDV Npro. (A) Immunoblot analysis of Flag- and HA-tandem-tagged Npro (FHNpro) (using anti-Flag antibody), IRF3, and TRIM56 expression in HeLa-FHNpro (lanes 1 and 2) and parental HeLa (lanes 3 and 4) cells. Where indicated, cells were treated with epoxomicin (100 nM) overnight prior to cell lysis. Asterisks indicate nonspecific bands, which demonstrate equal sample loading. (B) Protein complexes were purified from control HeLa and HeLa-FHNpro cells by anti-Flag affinity purification and fractionated on SDS-PAGE gels, followed by Coomassie blue staining. The protein bands for TRIM56, β-tubulin, and FH-Npro (identified later by mass spectrometry) are indicated on the right. (C) Co-IP analysis of the interaction of FH-Npro with V5-tagged human TRIM56 (TRIM56) and bovine TRIM56 (boTRIM56) in cotransfected 293FT cells. TRIM25-V5 was used as a negative control for the specificity of the Npro-TRIM56 interaction. Where indicated, cells were treated with epoxomicin overnight prior to cell lysis. Note that epoxomicin had no effect on the Npro-TRIM56/boTRIM56 associations. The input panel shows the immunoblotting of 1/10 of the whole-cell lysates used for IP. Note that ectopically expressed FH-Npro was detected as a doublet in immunoblot analyses of the whole-cell lysates. The upper band may represent monoubiquitinated FH-Npro. IB, immunoblot; Ig Hc, IgG heavy chain. (D) HeLaNpro-25 cells induced for Npro expression were transiently transfected with a vector encoding GFP-TRIM56 (left) or TRIM56-GFP (right) and subsequently fixed for immunostaining of Npro (using anti-Myc antibody). The subcellular localizations of GFP-tagged TRIM56 and Myc-Npro were examined by confocal microscopy.