Fig. 10.
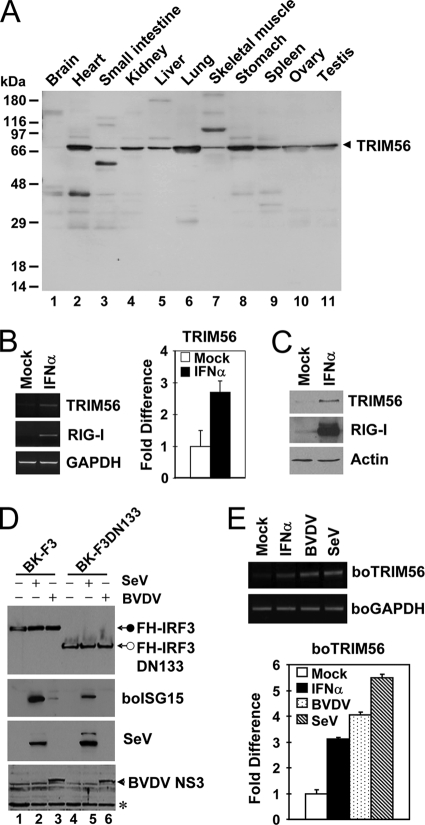
TRIM56 is ubiquitously expressed, and its expression is further upregulated by virus or IFN-α. (A) Expression of the TRIM56 protein in various human tissues detected by anti-TRIM56 antibody using Human Tissues INSTA-Blot (Calbiochem). (B) Semiquantitative RT-PCR (left) and real-time PCR (right) assessments of TRIM56 mRNA abundance in HeLa cells mock treated or stimulated with 500 U/ml of IFN-α for 16 h. RIG-I was used as a positive control for IFN treatment (left). (C) Immunoblot analysis of TRIM56 and RIG-I expression in THP1 cells mock treated or stimulated with IFN-α. Similar results were obtained with HeLa cells (data not shown). (D) Stable retroviral transduction of IRF3, but not that of IRF3 with an N-terminal 133-aa deletion (DN133), renders MDBK cells able to upregulate boISG15 expression following BVDV infection. Cells were mock treated (lanes 1 and 4) or infected with SeV (lanes 2 and 5) or with BVDV (lanes 3 and 6) for 12 h prior to cell lysis and immunoblot analysis of the ectopically expressed, Flag- and HA-tandem-tagged human IRF3 (FH-IRF3) or IRF3 DN133 (FH-IRF3DN133) (using anti-Flag antibody), bovine ISG15, SeV, and BVDV NS3. The asterisk indicates a nonspecific band, which demonstrates equal protein sample loading. (E) Semiquantitative RT-PCR (top) and real-time RT-PCR (bottom) analyses of boTRIM56 mRNA abundance in BK-F3 cells mock treated, stimulated with 500 U/ml of IFN-α, or infected with cp BVDV NADL (MOI of 0.1) or SeV (50 HAU/ml) for 16 h.