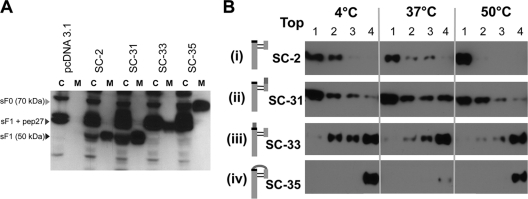
Fig. 9.
Efficient secretion of sF protein cleavage mutants and the requirement of cleavage at the furin site adjacent to the fusion peptide for liposome association. (A) Western blot analysis of the SC-2-derived cleavage site mutant produced from transfected 293T cells. The sF protein from cell lysates (C) and media (M) were stained with the 5His MAb. The C lanes represent 10-fold more cell equivalents than the M lanes. pcDNA3.1 is the empty vector control, and SC-2 is the positive-control parental sF protein. (B) Association of furin cleavage site sF mutants with liposomes. Each sF protein was incubated with liposomes in 10 mM HEPES buffer without imidazole at the indicated temperatures for 30 min. The mixtures were then treated with carbonate buffer, pH 11, for 10 min at 20°C to release any superficially bound sF protein before the flotation analysis. The mixtures were analyzed by using a flotation gradient as described in the legend to Fig. 7. The top fraction of the gradient is indicated. As indicated in the cartoons to the left of each panel, SC-2 is the parental protein, with both cleavage sites intact. In SC-31 the upstream cleavage site has been ablated, in SC-33 the downstream, fusion peptide-proximal site has been ablated, and in SC-35 both sites have been ablated.