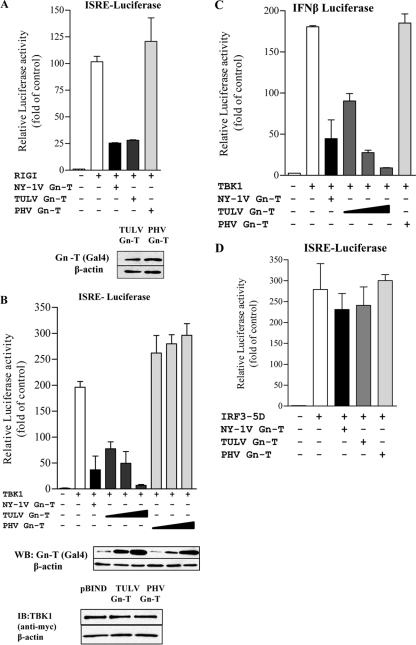
Fig. 3.
TULV Gn-T regulates RIG-I- and TBK1-directed ISRE and IFN-β transcription. HEK293 cells were transfected with an ISRE-driven luciferase reporter construct (A, B, and D) or an IFN-β-luciferase reporter construct (C) in the presence or absence of an activating (N)RIG-I (A), TBK1 (B and C), or IRF3-5D (D) expression vector. Cells were cotransfected with NY-1V Gn-T, PHV Gn-T, and TULV Gn-T (A and D) or increasing amounts of plasmid (0.5, 1, and 2 μg) expressing PHV Gn-T and TULV Gn-T (B and C) and the control empty vector (pBIND) to maintain constant DNA transfection levels. NY-1V Gn-T expression was used as a positive control. At 2 days posttransfection, cells were lysed, and luciferase activity was measured and normalized to Renilla luciferase levels. Luciferase activity is reported as the fold increase compared to that of controls lacking (N)RIG-I, TBK1, or IRF3-5D. Assays were performed in triplicate with similar results in at least three separate experiments. Western blot (WB) analysis indicates equal expression of TULV Gn-T and PHV Gn-T (A and B) and of TBK1 cotransfected with TULV Gn-T and PHV Gn-T (B). Cells were lysed at 48 h posttransfection, protein amounts were determined using bicinchoninic acid protein assay, and equal amounts were loaded onto a 10% SDS-PAGE gel. Proteins were detected by Western blotting using anti-Gal4 or anti-myc (Santa Cruz Biotechnology) and anti-mouse HRP-conjugated secondary antibody. Blots were stripped and reprobed with anti-β-actin (Sigma). IB, immunoblot.