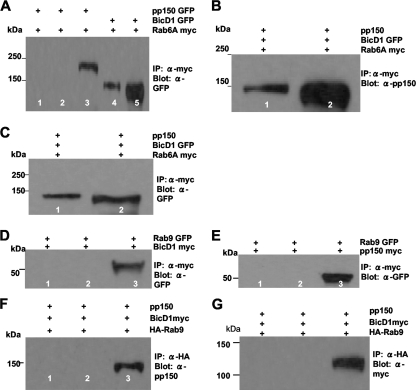
Fig. 3.
BicD1, pp150, and Rab6 form a ternary complex. (A) Coimmunoprecipitation (IP) of pp150 with Rab6A. Protein complexes from lysates of HEK 293 cells cotransfected with myc-tagged Rab6A and pp150-GFP (or Rab6A-myc and BicD1-GFP) were precipitated with anti-myc magnetic beads. Lane 1, negative control in which cell lysates were treated with unlabeled magnetic beads; lane 2, Rab6A-myc, which does not precipitate pp150-GFP; lane 3, pp150-GFP in the input lysate; lane 4, positive control showing that Rab6A-myc precipitates BicD1-GFP; lane 5, input lysate containing BicD1-GFP. The blot was probed with anti-GFP antibody. (B) Coimmunoprecipitation of pp150 and Rab6A in the presence of overexpressed BicD1. Protein complexes from lysates of HEK 293 cells cotransfected with Rab6A-myc, pp150, and BicD1-GFP were precipitated with anti-myc magnetic beads. Lane 1, Rab6A-myc precipitating pp150 when it was coexpressed with BicD1-GFP; lane 2, the input lysate containing pp150. The blot was probed with anti-pp150 MAb. (C) Protein complexes from lysates of HEK 293 cells cotransfected with Rab6A-myc, pp150, and BicD1-GFP (same as described for panel B) were precipitated with anti-myc magnetic beads. Lane 1, the precipitate is positive for BicD1-GFP; lane 2, input lysate containing BicD1-GFP. The blot was probed with anti-GFP antibody. (D) Coimmunoprecipitation of BicD1 with Rab9. Protein complexes from lysates of HEK 293 cells cotransfected with Rab9-GFP and BicD1-myc were precipitated with anti-myc magnetic beads. Lane 1, negative control in which cell lysates were treated with unlabeled magnetic beads; lane 2, BicD1-myc does not precipitate Rab9-GFP; lane 3, Rab9-GFP in the input lysate. The blot was probed with anti-GFP antibody. (E) Coimmunoprecipitation of pp150 with Rab9. Protein complexes from lysates of HEK 293 cells cotransfected with Rab9-GFP and pp150-myc were precipitated with anti-myc magnetic beads. Lane 1, negative control in which cell lysates were treated with unlabeled magnetic beads; lane 2, pp150-myc does not precipitate Rab9-GFP; lane 3, Rab9-GFP in the input lysate. The blot was probed with anti-GFP antibody. (F) Rab9 does not form a complex with BicD1 and pp150. Protein complexes from lysates of HEK 293 cells cotransfected with hemagglutinin (HA)-tagged Rab9, pp150 without a tag, and BicD1-myc were precipitated with anti-HA magnetic beads. Lane 1, negative control in which cell lysates were treated with unlabeled magnetic beads; lane 2, HA-Rab9 failing to precipitate pp150 when it was coexpressed with BicD1; lane 3, input lysate showing pp150. The blot was probed with anti-pp150 antibody. (G) Protein complexes from lysates of HEK 293 cells cotransfected with HA-Rab9, pp150, and BicD1-myc (same as described for panel F) were precipitated with anti-HA magnetic beads. Lane 1, negative control in which cell lysates were treated with unlabeled magnetic beads; lane 2, HA-Rab9 does not pull down BicD1; lane 3, input lysate showing BicD1. The blot was probed with anti-myc antibody.