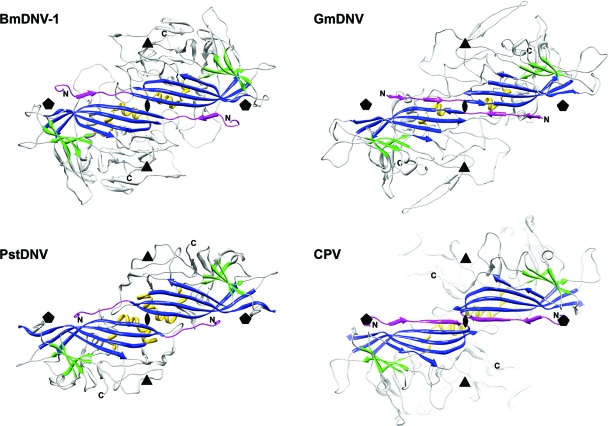
Fig. 2.
The spatial arrangement of the parvovirus core jelly roll and the N-terminal region for the capsid proteins of BmDNV-1, PstDNV, GmDNV, and CPV illustrate domain-swapping of the N terminus for the three known Densovirinae structures. Two 2-fold related symmetry mates are shown, viewed from the viral center along an icosahedral 2-fold axis. Conserved secondary structure elements of each protein subunit are colored blue (β-BIDG), green (β-CHEF), and gold (helical elements). The N-terminal region of the capsid protein, upstream of βB, including βA, is shown in magenta. The positions of icosahedral symmetry axes are indicated by polygonal symbols.