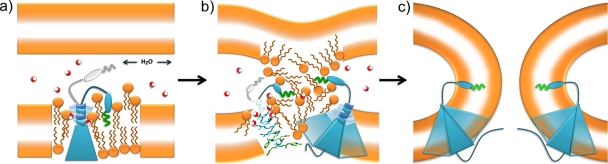
Fig. 8.
Model for the role of the p15 TMD in membrane fusion. (a) The p15 ectodomain, which contains an N-terminal myristic acid (green squiggle) and a proline-rich region (blue oval), and the TMD (blue triangle) alter lipid packing and membrane hydration (water molecules shown as red and white spheres). The β-branched residues and triserine motif (blue coil) at the N terminus of the p15 TMD are predicted to induce helix instability, with the triserine motif acting as a hydrogen bond donor to draw water molecules further into the bilayer, increasing vertical fluctuation of lipid molecules and expanding the thickness of the interfacial region. Reversible donor membrane associations of the p15 ectodomain and the flexible helix structure of the N terminus of the TMD are depicted in blue and gray. The combination of these activities poises the donor membrane for interaction with a closely apposed target membrane. (b) Close approach of the target membrane triggers lipid mixing, which is depicted as a hemifused lipid emulsion rather than an ordered hourglass structure to reflect potential dynamic changes to the structure and hydration of the outer leaflet induced by the combined effects of the ectodomain and TMD described in panel a. The left side shows a side view of the p15 TMD modeled as an α-helix. The side chains are colored blue or green (aromatic residues), and the oxygens on polar side chains are colored red. The juxtaposition of three p15 TMDs due to homomeric p15 interactions (a trimer is shown merely for illustrative purposes) is represented with translucence on the right side. (c) The association of multiple p15 monomers at the fusion site is depicted (represented with translucence). The β-branched residues and triserine motif are omitted for simplicity. The funnel-shaped architecture of the p15 TMD is predicted to induce positive curvature of the inner leaflet, promoting the rapid transition from lipid mixing to pore formation.