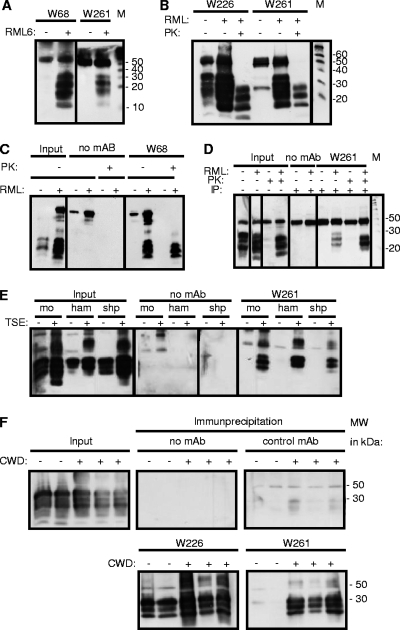
Fig. 1.
Reaction patterns of PrPSc-specific MAbs in immunoprecipitation (IP). (A) MAbs W68 and W261 react exclusively with the PrPSc isoform. (B and C) Treatment of precipitates with PK reveals the presence of PK-resistant PrPSc in MAb-precipitated material for W261 (B) and W68 (C). (D) Treatment of samples with PK prior to immunoprecipitation with MAb W261 reveals the interaction of W261 with the PK-resistant core of PrPSc. (E) (Right) Specific immunoprecipitation of scrapie PrPSc from mouse (mo), hamster (ham), and sheep (shp) samples with MAb W261. (Left) The presence of PrPC and PrPSc is shown in parallel. (F) Specific immunoprecipitation from brain samples of deer with CWD. The presence of PrP is demonstrated by the use of W226 (lower left). W261 reacts with CWD-affected brain samples but not with control brain samples, kindly provided by E. Hoover. Immunoprecipitations were performed using 200 μl of 1% brain homogenate, and complete supernatants of boiled precipitates were analyzed by Western blotting. For input material, 30 μl of 1% brain homogenate was loaded per lane. Western blots using mouse tissue were developed using antibody W226. Prion proteins on immune blots with human tissue were detected using MAb 6H4 (19). For the detection of prion proteins of animal species other than mouse, antibody POM-1 was used (34). The signal at 50 kDa corresponds to the heavy chain of mouse immunoglobulin.