Abstract
Acyclovir, a nucleoside analog, is thought to be specific for the human herpesviruses because it requires a virally encoded enzyme to phosphorylate it to acyclovir monophosphate. Recently, acyclovir triphosphate was shown to be a direct inhibitor of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. Here, we showed that acyclovir is an inhibitor of HIV-1 replication in CD4+ T cells from cord blood that have undetectable levels of the eight human herpesviruses. Additionally, acyclovir phosphates were detected by reverse-phase-high performance liquid chromatography (RP-HPLC) and quantified in a primer extension assay from cord blood. The data support acyclovir as an inhibitor of HIV-1 replication in herpesvirus-negative cells.
Acyclovir therapy for treatment of herpes simplex virus 2 (HSV-2) in patients coinfected with HSV-2 and human immunodeficiency virus type 1 (HIV-1) can potentially modulate the progression of HIV-1 disease. There is evidence of more rapid HIV-1 disease progression in dually infected individuals (6, 15, 29) as well as in vitro data demonstrating that HSV-2 proteins can activate the HIV-1 long terminal repeat, leading to increased HIV-1 replication (10, 18, 23). Recent trials of acyclovir monotherapy for HSV-2 infection in HSV-2/HIV-1-coinfected individuals showed an approximately 0.33 to 0.5 log10 decrease in HIV-1 plasma levels, but the overall benefits of this approach remain unclear (24, 30, 36). One study reported that acyclovir therapy in HSV-2/HIV-1-coinfected patients did not prevent transmission of HIV-1 to uninfected partners (4). Likewise, acyclovir prophylaxis in HSV-2-infected patients demonstrated no significant decrease in the acquisition of HIV-1 compared to that for patients taking a placebo (3, 33). In addition, the plasma load of HIV-1 is a strong indicator of disease progression (22), but despite the observed sustained decrease in HIV-1 load, recent data show no differences in viral load set points or CD4+ T cell counts in patients taking acyclovir at the time of HIV-1 acquisition from those of a control group of patients (14). Together, these results highlight the need for a better understanding of the effects of acyclovir treatment in HSV-2/HIV-1-coinfected patients, due to the high rate of coinfection (6).
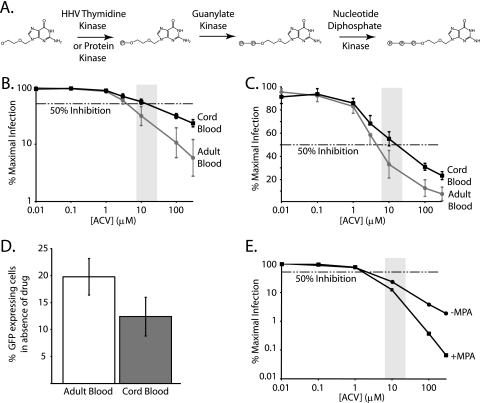
Acyclovir, (Fig. 1A), identified as an antiherpetic drug in the 1980s (27, 28), is converted intracellularly to the active 5′-triphosphate form that inhibits viral DNA polymerase. Acyclovir is specific for herpesviruses because it requires the virally encoded thymidine kinase (TK) to add the alpha phosphate to the analog, giving rise to acyclovir monophosphate (ACVMP), which is then sequentially phosphorylated by cellular enzymes to acyclovir triphosphate (ACVTP) (9, 12) (Fig. 1A). HSV-2 is not the only human herpesvirus (HHV) encoding an enzyme that phosphorylates acyclovir. All HHVs encode TK or a protein kinase that phosphorylates acyclovir (8). For example, the highly prevalent HHV type 6 (HHV-6) and HHV-7, T-lymphocyte-tropic viruses (5, 16), contain the U69 gene that for HHV-6 has been shown to encode the enzyme responsible for phosphorylating acyclovir (1). Early studies performed to assess intracellular concentrations of ACVTP in herpesvirus-infected and uninfected cell lines showed that even in the absence of viral TK, detectable levels of ACVTP were present, but at much lower levels (7, 32). Although it is generally accepted that a herpesvirus enzyme is required to phosphorylate acyclovir to ACVMP, this finding prompted additional exploration into possible cellular enzymes that could phosphorylate acyclovir. In 1985, Keller et al. (13) demonstrated that cellular cytoplasmic 5′ nucleotidase could phosphorylate acyclovir, while others (32) postulated that deoxycytidine kinase was the responsible enzyme. Because concentrations of ACVTP in infected cells were many times higher than in uninfected cells (32), the identity of the cellular kinase with acyclovir-phosphorylating activity was not pursued further.
Fig. 1.
Acyclovir is an anti-HIV-1 drug. (A) Enzymes that phosphorylate acyclovir to the active 5′-triphosphate form in HHV-infected cells. Shown is the prodrug acyclovir, a herpesvirus-specific nucleoside analog. The first phosphate is added to a hydroxyl that is structurally analogous to the 5′ end of a nucleoside. In HHV-infected cells, this reaction is carried out by a herpesvirus-encoded TK or protein kinase. The monophosphate is retained in the cell, and the second and third phosphates are sequentially added by the indicated cellular enzymes. (B) Acyclovir inhibits HIV-1 replication in a single-round infectivity assay using CD4+ T lymphoblasts from healthy adult peripheral blood and cord blood. Activated CD4+ T cells isolated from healthy adult or cord blood were infected with an HIV-1 pseudovirus in the presence of increasing concentrations of acyclovir. Dose response curves for adult (gray line and circle) and cord (black line and square) cells are plotted as percentages of maximal infection, defined for each cell type as the number of GFP-expressing cells in the presence of the drug divided by the number of GFP-expressing cells in the absence of a drug effect on a log-log graph. Error bars represent the standard errors of the means of the data from three replicate experiments with cells from six separate healthy or cord blood donors. The dashed horizontal line represents 50% inhibition. The lightly shaded box represents the peak clinical concentration range of acyclovir, with peak plasma concentrations ranging from 7 μM to 26.6 μM, depending on the form and dose. (C) Traditional semilog representation of data from panel B. The dose response curve was plotted from the same data as for panel B, graphed on a different scale to emphasize the anti-HIV activity of acyclovir at clinical concentrations. (D) Comparison of infectibilities of cord and adult cells. Bar graphs for adult (white) and cord (dark gray) cells represent the average percentages of GFP-expressing cells with infection in the absence of the drug, using the same number of viral particles per number of cells. The amounts of virus added for infection were equivalent for both adult and cord blood and were determined by a viral titration curve in adult cells. The volume of virus that yielded approximately 20% GFP-expressing cells was used for the dose response curves. (E) MPA enhances the anti-HIV activity of acyclovir. Acyclovir dose response curves generated in the absence (circle) or presence (square) of 5 μg/ml MPA as for panel A. In the absence of acyclovir, the levels of infection were the same in wells with and without MPA. ACV, acyclovir.
Recently, we and others (17, 21) showed that acyclovir can inhibit HIV-1 replication and that ACVTP is a substrate of HIV-1 reverse transcriptase (RT). One key finding from these studies was that the anti-HIV-1 activity of acyclovir is HSV-2 independent. Lisco et al. quantified levels of HHV in tonsil samples used in HIV-1 infection assays and found HHV-6 present in all samples where acyclovir inhibited HIV-1 replication (17). This finding suggests that coinfection with an HHV is necessary for the anti-HIV-1 activity of acyclovir.
To further study the herpesvirus dependence of acyclovir inhibition of HIV-1 replication, we used a single-round HIV-1 infectivity assay capable of detecting individual infection events in primary CD4+ T cells (19, 31, 35). We simultaneously quantified levels of the eight HHVs present in the CD4+ T lymphoblasts from peripheral blood and cord blood that were used as target cells in infectivity assays. We studied cord cells because of the high prevalence of HHV-6 and -7 infections in healthy adults (2). Greater than 90% of the population is infected with at least one of these viruses by age 2, but importantly, levels are low or undetectable in cord blood (11). This makes cord cells ideal for studying the requirement for herpesvirus infection to observe inhibition of HIV-1 replication by acyclovir.
To compare the anti-HIV-1 activities of acyclovir in adult peripheral blood and cord blood CD4+ T cells, we activated mononuclear cells for 48 to 72 h with phytohemagglutinin (PHA) and isolated CD4+ T cells. Cells were infected as described previously (19, 31, 35) with HIV-1 pseudovirions encoding green fluorescent protein (GFP). CD4+ T cells were preincubated for 16 to 20 h with increasing concentrations of acyclovir prior to being infected, and infection levels were evaluated 72 h later, using flow cytometry, by quantifying the number of GFP-positive cells. Results were plotted using a log-log plot (Fig. 1B) or linear-log plot (Fig. 1C) and showed that acyclovir inhibited HIV-1 infection similarly in both cell types. The V75I mutation in RT, which confers resistance to acyclovir in peripheral blood mononuclear cells (PBMCs) (20), also confers resistance in cord blood cells (data not shown). A dose-dependent inhibition of HIV replication by acyclovir was also observed in a multiround infection assay using adult peripheral blood CD4+ T cells (data not shown). The average 50% inhibitory concentration (IC50) in adult peripheral blood cells is ∼4 μM, while the average for cord blood cells is ∼21 μM. The IC50 is slightly higher for the cord samples, but activity is still within the clinical concentrations of acyclovir achieved in vivo (34) and could simply reflect differences in the activation state of the cells or the degree of HHV coinfection. The overall infectibility of cord cells in the absence of the drug was slightly lower than that of adult cells (Fig. 1D). Quantitative real-time PCR was performed to determine if known HHVs were present in CD4+ T lymphocytes used in the single-round infectivity assays. To do this, we isolated cellular DNA from ∼5 × 106 CD4+ T cells from each donor and used herpesvirus-specific primer and probe sets as reported by Lisco et al. (17) in individual PCR mixtures containing DNA from ∼150,000 to 250,000 CD4+ T cells. As shown in Table 1, all adult blood donors had detectable levels of the T-cell-tropic HHV-6 and -7, with HHV-7 being present in <0.3% of cells. Despite this low level of herpesvirus infection, acyclovir consistently inhibited HIV-1 replication in the single-round infectivity assay. Interestingly, none of the HHVs were detected in cord blood samples with our PCR, with the exception of sample 7, which contained 1 to 12 copies of HSV-1 DNA in ∼150,000 to 250,000 cells. One advantage of using flow cytometry to quantify HIV-1 infection events is the ability to collect thousands of events. For each dose response curve, ∼100,000 cells were infected, and ∼50,000 events were collected per drug concentration. The average percentage of cells infected in the absence of the drug was around 20%, or 10,000 cells. Combining the real-time PCR and infectivity results, our data suggest that at most, only 30 of 10,000 cells contain an HHV genome. Since the shape of the dose response curve (Fig. 1B) is similar to curves for other anti-HIV drugs, it appears that acyclovir can inhibit HIV-1 infection in cells that lack an HHV genome.
Table 1.
Quantification of HHV levels in CD4+ T lymphoblasts from adult peripheral and cord blood
| Blood sourcea | Donor | No. of HHV copies/sample for indicated virusb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HHV-1 | HHV-2 | HHV-3 | HHV-4 | HHV-5 | HHV-6 | HHV-7 | HHV-8 | ||
| Adult | 1 | <1 | <3 | <1 | <10 | <3 | 0.4–1 | 3–84 | <100 |
| 2 | <1 | <3 | <1 | <10 | <3 | 1 | 15–53 | <100 | |
| 3 | <1 | <3 | <1 | <10 | <1 | 1 | 191–493 | <100 | |
| 4 | <1 | <3 | <1 | <10 | <1 | 1 | 161–212 | <100 | |
| 5 | <1 | <3 | <30 | <10 | <1 | 0.3–3 | 35–76 | <100 | |
| Cord | 6 | <1 | <3 | <30 | <10 | <1 | <1 | <30 | <100 |
| 7 | 12 | <3 | <3 | <30 | <1 | <1 | <10 | <30 | |
| 8 | <1 | <3 | <3 | <30 | <1 | <1 | <10 | <30 | |
| 9 | <1 | <1 | <1 | <10 | <1 | <1 | <10 | <10 | |
| 10 | <1 | <3 | <30 | <10 | <3 | <30 | <1 | <300 | |
CD4+ T cells were obtained from adult peripheral blood or cord blood.
Copy numbers of HHV genomes were determined by quantitative real-time PCR in triplicate as described previously (17), using input DNA from 150,000 to 250,000 CD4+ T cells/sample. A value preceded by the symbol for “less than” (<) represents the maximum possible number of copies. Ranges represent values from determinations in triplicate. HHV-1, human herpes simplex virus 1; HHV-2, human herpes simplex virus 2; HHV-3, varicella-zoster virus; HHV-4, Epstein-Barr virus; HHV-5, human cytomegalovirus; HHV-8, Kaposi's sarcoma-associated herpesvirus.
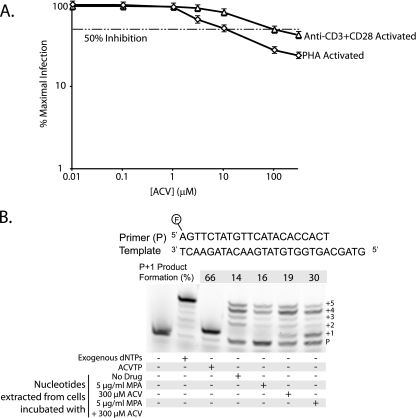
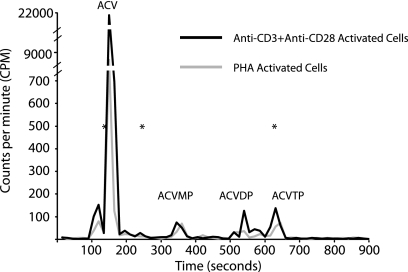
To further explore the ability of acyclovir to inhibit HIV-1 replication in CD4+ T cells from cord blood, we compared two methods of activation, the approach described above using PHA and a second method using beads conjugated with anti-CD3 plus anti-CD28. For anti-CD3 plus anti-CD28 activation, CD4+ T cells were purified prior to being activated. As shown in Fig. 2A, representative dose response curves from cord sample 10 demonstrate similar results with the two different activation methods. The IC50 for cells activated by anti-CD3 plus anti-CD28 is higher than that for PHA-activated cells, but nevertheless, inhibition by acyclovir is apparent. We sought to further confirm the presence of ACVTP in cord blood cells. To do this, we incubated 1 × 106 PHA-activated CD4+ T cells from cord blood with 300 μM acyclovir, 5 μg/ml mycophenolic acid (MPA), or a combination of both. MPA decreases cellular levels of dGTP by inhibiting de novo purine biosynthesis (26). Acyclovir is a deoxyguanosine analogue, and MPA increases the levels of ACVTP relative to those of dGTP, thereby potentiating the anti-HSV effect of acyclovir in vitro and in vivo (25). After 24 h, cells were lysed with 60% methanol (MeOH) and extracts were reconstituted in 50 mM Tris-HCl [pH 8.0]–10 mM MgCl2 and used as the sole source of nucleotides for a primer extension assay with purified HIV-1 RT (21). Primer extension products were separated by electrophoresis and visualized by fluorescence (Fig. 2B). We quantified the primer plus 1 extended product because all products larger than the plus 1 band reflect nucleotide incorporation not inhibited by ACVTP and show that this band intensity is increased with coincubation of 300 μM acyclovir and 5 μg/ml MPA, suggesting increased ACVTP incorporation (Fig. 2B, lane 7). This finding is consistent with infectivity data for adult CD4+ T cells, where we observed enhanced inhibition of HIV replication in the presence of both MPA and acyclovir compared to that with acyclovir alone (Fig. 1E). To directly demonstrate the presence of ACVTP in these cells, we used reverse-phase-high performance liquid chromatography (RP-HPLC) to detect acyclovir phosphates. Cells (15 × 106) from cord blood sample 10 activated by PHA or anti-CD3 plus anti-CD28 were incubated with 20 μCi [3H]acyclovir overnight. Cells were lysed, and extracts were reconstituted in water. Phosphates of acyclovir were separated by ion pairing RP-HPLC on a C18 beta basic column (3 × 250 mm; 5 μm) using a 15-min gradient elution with 7.5% acetonitrile and 4 mM tetrabutylammonium bromide in 100 mM phosphate buffer (pH 6) for 5 min, increased to 30% acetonitrile over min 5 to 6, maintained at 30% acetonitrile for 8 min, and reduced to 7.5% acetonitrile for min 14 to 15. Fractions were collected every 15 s. As shown in Fig. 3, ACVMP, acyclovir diphosphate (ACVDP), and ACVTP were detected in both samples even though no HHV DNA was detectable (Table 1). A similar pattern was observed in extracts from ACV-treated HEK293 cells that were transfected with a herpes simplex virus 1 thymidine kinase expression vector (data not shown).
Fig. 2.
Inhibition of HIV-1 replication and detection of ACVTP in herpesvirus-negative CD4+ T cells from cord blood. (A) Acyclovir inhibits HIV-1 replication in cord blood CD4+ T cells activated by either PHA or anti-CD3 plus anti-CD28. Dose response curves for cord blood cells activated by PHA (diamonds) or anti-CD3 plus anti-CD28 (triangles) are plotted on a log-log plot as described in the legend to Fig. 1B. Curves are representative plots from cord sample 10. The dashed horizontal line represents 50% inhibition. Error bars that would be obscured by the symbols are drawn at the edges of the symbols. (B) Reverse transcriptase assays were performed as previously described (21). For all lanes, 100 nM of the indicated 5′-fluorescein-labeled primer and 200 nM template were incubated with 1 μM RT for 30 min to 1 h. Lane 1, the negative control, contains primer/template and RT; lane 2 contains 10 μM deoxynucleoside triphosphates (dNTPs); and lane 3 contains 10 μM ACVTP. Lanes 4 through 7 contain nucleotides isolated from approximately 200,000 cells incubated under various conditions. Lane 4 cells were incubated without the drug, lane 5 cells with 5 μg/ml MPA, lane 6 cells with 300 μM acyclovir, and lane 7 cells with 300 μM acyclovir and 5 μg/ml MPA. The percent primer plus 1 (P+1) product formation was quantified using ImageJ software and is equal to the signal intensity of the plus 1 (+1) band as a fraction of the total signal intensity of all bands per lane.
Fig. 3.
Detection of acyclovir analytes using RP-HPLC. Approximately 15 × 106 cells were incubated overnight with 20 μCi [3H]acyclovir (specific activity, 15.3 Ci/mmol), and analytes were separated by applying the concentrated cell lysate to a C18 beta basic column and collecting fractions every 15 s. The data are from cord sample 10. Tritium counts from extracts of anti-CD3-plus-anti-CD28-activated cells (solid black line) and PHA-activated cells (solid gray line) are graphed as functions of elution time. Pure standards of acyclovir, ACVMP, and ACVTP (Moravek) in 100% MeOH were mixed together to obtain the retention time of each analyte. Retention times of standards were used to identify peaks and are represented by asterisks corresponding to acyclovir (137 s), ACVMP (245 s), and ACVTP (627 s). No standard for ACVDP was available, and the identity of this peak is inferred.
An understanding of the inhibitory potential of acyclovir against HIV-1 replication is important because of the extensive use of acyclovir in HSV-2/HIV-1-coinfected patients (24, 33). The data presented here suggest that direct inhibition of HIV-1 infectivity by acyclovir may also be independent of HHVs. Clinical trials of acyclovir have been aimed at HSV-2/HIV-1-coinfected patients, and clinical trials of acyclovir in HIV-1-infected individuals not coinfected with herpes simplex viruses are now under way. Our results suggest that these patients are likely to experience the same modest reduction in HIV-1 load as seen in HSV-2/HIV-1-coinfected patients. Studies to identify the non-HHV kinase that phosphorylates acyclovir should provide additional understanding of the activation of acyclovir into an anti-HIV-1 drug and may contribute to the development of more potent acyclovir analogues.
Acknowledgments
We thank Craig Hendrix and the Clinical Pharmacology Analytical Laboratory for providing reagents, protocols, and equipment for RP-HPLC and James Stivers for input on experiments described in the manuscript.
This work was supported by NIH grant AI081600 and by the Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 16 February 2011.
REFERENCES
- 1. Ansari A., Emery V. C. 1999. The U69 gene of human herpesvirus 6 encodes a protein kinase which can confer ganciclovir sensitivity to baculoviruses. J. Virol. 73:3284–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boutolleau D., et al. 2005. Detection of human herpesvirus 7 DNA in peripheral blood reflects mainly CD4+ cell count in patients infected with HIV. J. Med. Virol. 76:223–228 [DOI] [PubMed] [Google Scholar]
- 3. Celum C., et al. 2008. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 371:2109–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Celum C., et al. 2010. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N. Engl. J. Med. 362:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark D. A., Freeland M. L., Mackie L. K., Jarrett R. F., Onions D. E. 1993. Prevalence of antibody to human herpesvirus 7 by age. J. Infect. Dis. 168:251–252 [DOI] [PubMed] [Google Scholar]
- 6. Corey L., Wald A., Celum C. L., Quinn T. C. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 35:435–445 [DOI] [PubMed] [Google Scholar]
- 7. Datta A. K., Pagano J. S. 1983. Phosphorylation of acyclovir in vitro in activated Burkitt somatic cell hybrids. Antimicrob. Agents Chemother. 24:10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Clercq E., et al. 2001. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev. Med. Virol. 11:381–395 [DOI] [PubMed] [Google Scholar]
- 9. Elion G. B., et al. 1977. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl. Acad. Sci. U. S. A. 74:5716–5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Golden M. P., et al. 1992. Activation of human immunodeficiency virus by herpes simplex virus. J. Infect. Dis. 166:494–499 [DOI] [PubMed] [Google Scholar]
- 11. Hall C. B., et al. 1994. Human herpesvirus-6 infection in children. A prospective study of complications and reactivation. N. Engl. J. Med. 331:432–438 [DOI] [PubMed] [Google Scholar]
- 12. Keller P. M., et al. 1981. Enzymatic phosphorylation of acyclic nucleoside analogs and correlations with antiherpetic activities. Biochem. Pharmacol. 30:3071–3077 [DOI] [PubMed] [Google Scholar]
- 13. Keller P. M., McKee S. A., Fyfe J. A. 1985. Cytoplasmic 5′-nucleotidase catalyzes acyclovir phosphorylation. J. Biol. Chem. 260:8664–8667 [PubMed] [Google Scholar]
- 14. Kim H. N., et al. 2010. Effect of acyclovir on HIV-1 set point among herpes simplex virus type 2-seropositive persons during early HIV-1 infection. J. Infect. Dis. 202:734–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krone M. R., et al. 2000. Herpes simplex virus type 2 shedding in human immunodeficiency virus-negative men who have sex with men: frequency, patterns, and risk factors. Clin. Infect. Dis. 30:261–267 [DOI] [PubMed] [Google Scholar]
- 16. Levy J. A., Ferro F., Greenspan D., Lennette E. T. 1990. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet 335:1047–1050 [DOI] [PubMed] [Google Scholar]
- 17. Lisco A., et al. 2008. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe 4:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Margolis D. M., Rabson A. B., Straus S. E., Ostrove J. M. 1992. Transactivation of the HIV-1 LTR by HSV-1 immediate-early genes. Virology 186:788–791 [DOI] [PubMed] [Google Scholar]
- 19. McMahon M. A., et al. 2007. The HBV drug entecavir—effects on HIV-1 replication and resistance. N. Engl. J. Med. 356:2614–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McMahon M. A., Siliciano J. D., Kohli R. M., Siliciano R. F. 2010. Sensitivity of V75I HIV-1 reverse transcriptase mutant selected in vitro by acyclovir to anti-HIV drugs. AIDS 24:319–323 [DOI] [PubMed] [Google Scholar]
- 21. McMahon M. A., et al. 2008. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J. Biol. Chem. 283:31289–31293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mellors J. W., et al. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167–1170 [DOI] [PubMed] [Google Scholar]
- 23. Mosca J. D., et al. 1987. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc. Natl. Acad. Sci. U. S. A. 84:7408–7412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagot N., et al. 2007. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N. Engl. J. Med. 356:790–799 [DOI] [PubMed] [Google Scholar]
- 25. Neyts J., Andrei G., De Clercq E. 1998. The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpesvirus activities of acyclovir, ganciclovir, and penciclovir in vitro and in vivo. Antimicrob. Agents Chemother. 42:216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nimmesgern E., Fox T., Fleming M. A., Thomson J. A. 1996. Conformational changes and stabilization of inosine 5′-monophosphate dehydrogenase associated with ligand binding and inhibition by mycophenolic acid. J. Biol. Chem. 271:19421–19427 [DOI] [PubMed] [Google Scholar]
- 27. Reardon J. E. 1989. Herpes simplex virus type 1 and human DNA polymerase interactions with 2′-deoxyguanosine 5′-triphosphate analogues. Kinetics of incorporation into DNA and induction of inhibition. J. Biol. Chem. 264:19039–19044 [PubMed] [Google Scholar]
- 28. Reardon J. E., Spector T. 1989. Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J. Biol. Chem. 264:7405–7411 [PubMed] [Google Scholar]
- 29. Schacker T., et al. 1998. Famciclovir for the suppression of symptomatic and asymptomatic herpes simplex virus reactivation in HIV-infected persons. A double-blind, placebo-controlled trial. Ann. Intern. Med. 128:21–28 [DOI] [PubMed] [Google Scholar]
- 30. Schacker T., Zeh J., Hu H., Shaughnessy M., Corey L. 2002. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J. Infect. Dis. 186:1718–1725 [DOI] [PubMed] [Google Scholar]
- 31. Shen L., et al. 2008. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat. Med. 14:762–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smee D. F., Boehme R., Chernow M., Binko B. P., Matthews T. R. 1985. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem. Pharmacol. 34:1049–1056 [DOI] [PubMed] [Google Scholar]
- 33. Watson-Jones D., et al. 2008. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N. Engl. J. Med. 358:1560–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weller S., et al. 1993. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin. Pharmacol. Ther. 54:595–605 [DOI] [PubMed] [Google Scholar]
- 35. Zhang H., et al. 2004. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J. Virol. 78:1718–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zuckerman R. A., et al. 2007. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J. Infect. Dis. 196:1500–1508 [DOI] [PubMed] [Google Scholar]