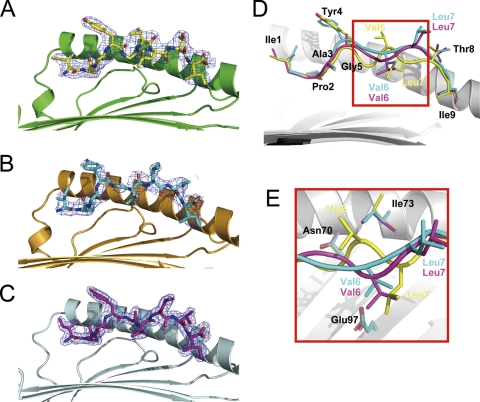
Fig. 5.
Two distinct conformations of peptides presented by N*01801. (A to C) Electron density at the 1σ contour level clearly shows the peptide conformations of the bovine MHC-specific epitope within molecule 1 (yellow) (A), molecule 2 (cyan) (B), and N*01801-Mβ (purple) (C). (D) Peptide alignment according to the superposition of the α1α2 domains of molecule 1, molecule 2, and Mβ represents two different conformations of IPA. One is the “double-M” conformation of peptide IPA-M1 in molecule 1 (yellow), with Gly5 and Leu7 located deep in the groove and the side chain of Val6 protruding out of the groove. The other is that of molecule 2 (IPA-M2) (cyan) and Mβ (IPA-Mβ) (purple), which have similar M-shaped conformations, with Val6 as a secondary anchor residue in the middle of the peptide and the side chain of Leu7 solvent exposed. (E) This phenomenon is associated mainly with the conformational change of Ile73 in the α1 domain of N*01801. In molecule 1, Ile73 (yellow) points upward, creating space for the middle of the peptide (yellow) of molecule 1 to bulge out of the groove. In contrast, Ile73 of molecule 2 (cyan) or Mβ (purple) protrudes toward the groove and suppresses the middle of the peptide of molecule 2 (cyan) or Mβ (purple) down toward the groove.