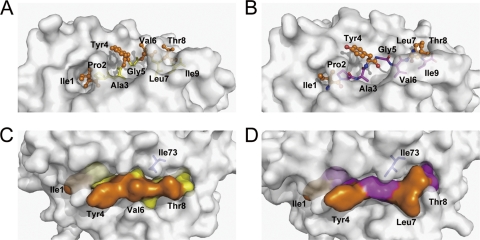
Fig. 6.
Exposed area of peptide IPA presented by bovine MHC I N*01801. (A) Peptide IPA-M1 presentation in the groove of N*01801 molecule 1. The residues exposed to the solvent are highlighted by an orange ball-and-stick representation (Ile1, Tyr4, Val6, and Thr8). The other residues, shown as yellow sticks, are located deep in the groove and act as anchors. (B) Molecule 2 (IPA-M2) and N*01801-Mβ (IPA-Mβ) present the IPA peptide (N*01801-Mβ shown here) in a different way than molecule 1. Leu7 rather than Val6 (as in molecule 1) protrudes out of the groove. Here, Val6 acts as a secondary anchor residue. (C) The exposed area of IPA-M1 in molecule 1 is largely covered by residues Tyr4, Val6, and Thr8. Residue Ile73 (blue) of the N*01801 heavy chain points toward the solvent, leaving space in the middle of the peptide exposed. (D) For molecule 2 and N*01801-Mβ (N*01801-Mβ shown here), the IPA peptide exposes Tyr4, Leu7, and Thr8 for potential TCR docking. Residue Ile73 (blue) hangs over the main chain of the peptide and pushes Val6 into the groove.