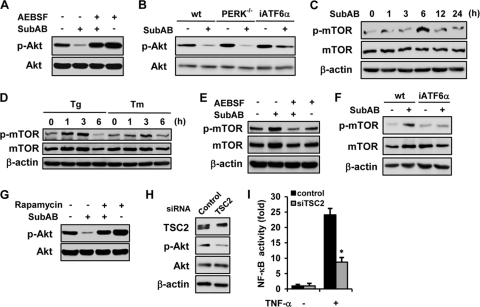
Fig. 5.
Suppression of Akt phosphorylation by SubAB through the ATF6-Akt-mTOR pathway. (A) NRK-52E cells were treated with or without SubAB for 24 h in the absence or presence of AEBSF and subjected to Western blot analysis of phosphorylated Akt. (B) Wild-type MEF (wt), PERK-knockout MEF (PERK−/−), and iATF6α MEF were treated with or without SubAB and subjected to Western blot analysis. NRK-52E cells were treated with SubAB (C) or thapsigargin or tunicamycin (D) for indicated time periods and subjected to Western blot analysis of phosphorylated mTOR. Levels of total mTOR and β-actin were used as loading controls. (E) Cells were treated with or without SubAB for 3 h in the absence or presence of AEBSF and subjected to Western blot analysis. (F) Wild-type MEF and iATF6α MEF were treated with or without SubAB and subjected to Western blot analysis of phosphorylated mTOR. (G) NRK-52E cells were pretreated with or without 100 nM rapamycin for 30 min, exposed to SubAB for 24 h, and subjected to Western blot analysis of phosphorylated Akt. (H) Western blot analysis of TSC2 and phosphorylated Akt in cells transiently transfected with control siRNA or TSC2 siRNA. (I) Cells were transiently cotransfected with pNFκB-Luc together with control siRNA or TSC2 siRNA. After 48 h, cells were treated with or without TNF-α for 6 h and subjected to luciferase assay.