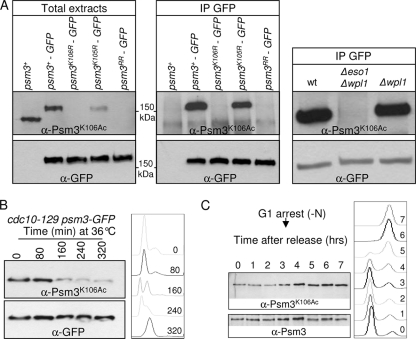
Fig. 3.
Eso1-dependent Psm3K106 acetylation during the cell cycle. (A) The anti-Psm3K106Ac antibody reacts with Psm3 in a K106-dependent manner in total extracts (left) and GFP immunoprecipitates (middle). (Right) Psm3 detection by anti-Psm3K106Ac antibodies is dependent on Eso1, but not on Wpl1. (B and C) Psm3K106 acetylation during the cell cycle. (B) Cells bearing the thermosensitive mutation cdc10-129 were shifted to the restrictive temperature to induce G1 arrest. Samples were taken at regular time intervals after the temperature shift to monitor Psm3K106Ac on total protein extracts as the cells progressed from an asynchronous population (time zero) to a homogeneous G1 arrest. Flow cytometry analysis of the DNA content (right) showed that Psm3K106Ac started to decrease as the G1 peak appeared. The drift in the G1 peak to the right at later time points was due to an increase in the mitochondrial DNA content as the cells elongated (47). (C) Cells were arrested in G1 by nitrogen deprivation and released synchronously into the cell cycle. Psm3K106Ac probed from total protein extracts was weakest in cells with a G1 DNA content and began to rise at the time of DNA replication. Anti-Psm3 antibodies were used to adjust protein loading so that similar amounts of Psm3 were present in all samples.