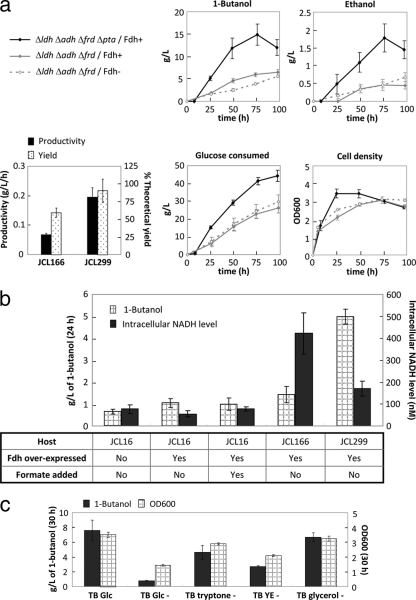
Fig. 3.
(a) Effects of Fdh overexpression and Pta deletion on anaerobic 1-butanol production. Time courses of alcohol production, cell growth, and glucose consumption are shown. A much higher yield and productivity of 1-butanol was achieved in JCL299 than in JCL166. It is important to note that other components present in the TB medium (such as yeast extracts) also contributed slightly to the 1-butanol titer, therefore affecting the yield. Solid black lines (Δldh Δadh Δfrd Δpta/Fdh+) refer to JCL299 transformed with plasmids pEL11, pIM8, and pCS138. Solid gray lines (Δldh Δadh Δfrd/Fdh+) represent JCL166 transformed with plasmids pEL11, pIM8, and pCS138. Dashed gray lines (Δldh Δadh Δfrd/Fdh−) refer to JCL166 transformed with plasmids pEL11 and pIM8. “Time” indicates time since inoculation. JCL166, BW ΔldhA ΔadhE ΔfrdBC; JCL299, BW ΔldhA ΔadhE ΔfrdBC Δpta. (b) Comparison of intracellular NADH levels and anaerobic 1-butanol production titers in the wild type and the engineered strains. All strains contained plasmids pEL11 and pIM8. Strains indicated as “Fdh over-expressed” also carry plasmid pCS138. A concentration of 20 mM formate was fed to the culture at the time of anaerobic switch where noted. The intracellular NADH level was measured using crude extracts prepared from the production culture after 24 h of fermentation. JCL16, wild type; JCL166, BW ΔldhA ΔadhE ΔfrdBC; JCL299, BW ΔldhA ΔadhE ΔfrdBC Δpta. (c) Medium analysis for anaerobic 1-butanol production. Fermentations of strain JCL299 harboring plasmids pEL11, pIM8, and pCS138 were performed in different medium compositions as indicated on the x axis (“−” indicates the absence of the particular component). The contribution of every element in the TB medium to 1-butanol production was analyzed by the subtraction of each component one by one (Glc, glucose; YE, yeast extract). Samples were taken after 30 h of fermentation. Cell densities are listed on the right y axis. Error bars show standard deviations. L, liter.