Abstract
This study aimed at investigating the antifungal activity of Wickerhamomyces anomalus and sourdough lactic acid bacteria to extend the shelf life of wheat flour bread. The antifungal activity was assayed by agar diffusion, growth rate inhibition, and conidial germination assays, using Penicillium roqueforti DPPMAF1 as the indicator fungus. Sourdough fermented by Lactobacillus plantarum 1A7 (S1A7) and dough fermented by W. anomalus LCF1695 (D1695) were selected and characterized. The water/salt-soluble extract of S1A7 was partially purified, and several novel antifungal peptides, encrypted into sequences of Oryza sativa proteins, were identified. The water/salt-soluble extract of D1695 contained ethanol and, especially, ethyl acetate as inhibitory compounds. As shown by growth inhibition assays, both water/salt-soluble extracts had a large inhibitory spectrum, with some differences, toward the most common fungi isolated from bakeries. Bread making at a pilot plant was carried out with S1A7, D1695, or a sourdough started with a combination of both strains (S1A7-1695). Slices of the bread manufactured with S1A7-1695 did not show contamination by fungi until 28 days of storage in polyethylene bags at room temperature, a level of protection comparable to that afforded by 0.3% (wt/wt) calcium propionate. The effect of sourdough fermentation with W. anomalus LCF1695 was also assessed based on rheology and sensory properties.
INTRODUCTION
The major problem for long-term shelf life of baked goods is contamination with fungi. Although they are used as the main chemical preservatives, salts of propionic, sorbic, and benzoic acids have shown several drawbacks. Reports on the appearance of cancer-like tumors in rats fed with propionic acid at concentrations of up to 4% led to prohibition of the use of calcium propionate in some European countries (31). Prolonged preservation of breads with high concentrations of calcium propionate stimulated the growth of resistant strains of Penicillium roqueforti (42). Since a few years ago, the European directive on preservatives (18) decreased the permitted concentrations of sorbate and propionate to 0.2 and 0.3% (wt/wt), respectively. Ethanol is also permitted as an antifungal preservative (2% [wt/wt]). Its inhibitory effect varies depending on the fungal species, and in some cases, it is not sufficient to prevent contamination (14).
Several studies have focused on the antifungal activity of compounds from natural sources and on biopreservation as effective alternatives to chemical preservatives. Several antimicrobial compounds have been identified, especially proteins or peptides from plants, as well as metabolites derived from fermentation by lactic acid bacteria (12, 23, 25, 42, 43). Besides acidification during sourdough fermentation, lactic acid bacteria also synthesize a wide range of low-molecular-weight compounds (27), peptides (28), and proteins (10, 37) with antifungal activity. Unfortunately, only a few studies have assessed their inhibitory activity under bread-making conditions. Recent studies proposed a combined effect of sourdough fermentation and extracts from plants which extended the shelf life of bread. The use of the water-soluble extract of Phaseolus vulgaris cv. Pinto together with sourdough fermentation by Lactobacillus brevis AM7 delayed mold growth during 21 days of storage at room temperature (10). The water-soluble extract of amaranth seeds was used as an ingredient for the manufacture of gluten-free and wheat flour breads, and inhibitory activity was achieved during long-term storage under pilot plant conditions (40). Recently, breads enriched with sourdough-fermented wheat germ delayed fungal growth for at least 28 days (37).
Besides these promising results, further efforts seem to be needed to achieve a long shelf life for leavened baked goods by use of convenient and simple biotechnology. Biopreservation using combinations of yeasts and bacteria has already been suggested as an alternative for storage of fruits and cereal grains (34). The capacity of antagonistic yeasts to control fungal contamination has been studied extensively, and several species, such as Candida oleophila, Cryptococcus albidus, and Metschnikowia fruticola, are included in commercially available biocontrol products (18). The mechanism for fungal inhibition varies depending on the species of yeast, features of the target fungi, and physical parameters of the biocontrol system (30, 34). Species belonging to the genus Pichia (e.g., Pichia guilliermondii, Pichia angusta, and Pichia anomala) are well known to control a range of postharvest fungi and to decrease sporulation and mycotoxin production (32). In particular, P. anomala (Wickerhamomyces anomalus, according to the latest taxonomic system) was shown to inhibit the growth of fungi in airtight-stored cereal grains (32). The biocontrol effect in a model grain silo with moist wheat (water activity of 0.96) was attributed to the antifungal action of metabolites derived from glycolysis rather than to competition for nutrients or activity of cell wall lytic enzymes (16).
This work aimed at investigating the mechanism of inhibition and the antifungal effect of a mixed starter consisting of selected yeasts and lactic acid bacteria during sourdough fermentation. A protocol for making bread with an extended shelf life was set up.
MATERIALS AND METHODS
Microorganisms, culture media, and growth conditions.
Lactobacillus brevis AM7 (previously selected for antifungal activity) (10) and 40 strains of sourdough lactic bacteria belonging to Lactobacillus rossiae (6 strains), Lactobacillus sanfranciscensis (9 strains), Lactobacillus plantarum (6 strains), Lactobacillus paraplantarum (1 strain), Lactobacillus pentosus (2 strains), Lactobacillus paralimentarius (2 strains), L. brevis (5 strains), Lactobacillus curvatus (2 strains), Lactobacillus sakei (1 strain), Lactobacillus fermentum (1 strain), Lactobacillus hilgardii (1 strain), Lactobacillus alimentarius (1 strain), Lactobacillus fructivorans (1 strain), Lactobacillus farciminis (1 strain), and Lactobacillus frumenti (1 strain) were from the Culture Collection of the Department of Biologia e Chimica Agro-Forestale ed Ambientale (University of Bari, Italy). W. anomalus LCF1694 and W. anomalus LCF1695 were from the personal culture collection of G. Cardinali (Dipartimento di Biologia Applicata, University of Perugia, Italy). Lactic acid bacteria were propagated for 24 h at 30°C in MRS (Oxoid Laboratories, Hampshire, United Kingdom) with the addition of fresh yeast extract (5% [vol/vol]) and 28 mM maltose to a final pH of 5.6 (mMRS). Yeasts were propagated for 48 h at 30°C in YEPG (10 g/liter yeast extract, 10 g/liter peptone, 20 g/liter glucose). Enumeration of lactic acid bacteria and yeasts in dough was carried out by plating on mMRS and YEPG, respectively, at 30°C for 48 h.
Penicillium roqueforti DPPMAF1 was used as the indicator microorganism for antifungal assays, since it corresponds to one of the more resistant fungi to chemical preservatives (10). Penicillium polonicum CBS 112490, Penicillium chrysogenum CBS 111214, Penicillium paneum CBS 101032, Penicillium albocoremium CBS 109582, Penicillium chermesinum CBS 117279, Penicillium carneum CBS 112297, Eurotium herbariorum CBS 117336, Eurotium rubrum CBS 150.92, Aspergillus parasiticus CBS 971.97, Aspergillus versicolor CBS 117286, and Penicillium bialowiezense CBS 110102 were from the culture collection of the Centraalbureau voor Schimmelcultures (Utrecht, Netherlands). All of the above species correspond to some of the most relevant spoilage fungi in baked goods (23). Fungi were grown on potato dextrose agar (pH 5.6) (PDA; Oxoid Laboratories) at 25°C for 24 to 72 h.
Wheat flour hydrolysate (WFH), used for the determination of conidial germination, was produced as described previously (19). WFH was chosen as the substrate because it is representative of the chemical composition of wheat flour.
Dough fermentation.
The characteristics of the flour (Triticum aestivum cv. Appulo) used were as follows: moisture, 14.2%; protein (N × 5.70), 11.5% of dry matter (d.m.); fat, 1.6% of d.m.; ash, 0.6% of d.m.; and total soluble carbohydrates, 1.5% of d.m.
Preliminarily, strains of lactic acid bacteria and yeasts were used singly for dough fermentation. Cells were cultivated until the late exponential phase of growth was reached. After growth, cells were harvested by centrifugation at 10,000 × g for 10 min at 4°C and were washed with 20 mM phosphate buffer, pH 7.0. Two hundred grams of flour, 115 ml of tap water, and 5 ml of the cell suspension (final cell density in the dough of ca. 108 and 106 CFU/g for lactic acid bacteria and yeasts, respectively) were used to produce 320 g dough (dough yield of 160) by use of a continuous high-speed mixer (60 × g; dough mixing time, 5 min). A sourdough containing both Lactobacillus plantarum 1A7 and W. anomalus LCF1695 (final cell density in the dough of ca. 108 and 106 CFU/g, respectively) was also manufactured (S1A7-1695). Dough fermentation was carried out at 30°C for 16 h. After fermentation, doughs were subjected to water/salt-soluble extraction or used directly for bread making. Fermentations were carried out in triplicate and analyzed twice.
Water/salt-soluble extracts.
Water/salt-soluble extracts from doughs were prepared according to the method originally described by Osborne (29) and modified by Weiss et al. (48). The supernatant was used for antifungal assay. The concentrations of peptides and proteins of the water/salt-soluble extracts and related fractions were determined by the o-phthaldialdehyde (OPA) and Bradford methods (6, 9), respectively.
Assays of antifungal activity.
Three different methods were used to determine the antifungal activity. The agar diffusion assay was carried out as described by Coda et al. (10). Petri plates (90-mm diameter) containing 10 ml of PDA (Oxoid) were inoculated with fungus. The plates were incubated at 25°C for 72 h. After the mycelial colony had developed, sterile blank paper disks (0.5-cm diameter) were placed at a distance of ca. 0.5 cm away from the rim of the mycelial colony. Ten microliters of each sample to be assayed was added to the disks. Plates were incubated at 25°C for 72 h until the mycelial growth overlaid the negative-control paper disk (without sample addition). At the same time, zones of inhibition were evident in corresponding paper disks containing samples with inhibitory activity.
The inhibitory spectrum of the water/salt-soluble extracts was assayed based on the hyphal radial growth rates of fungi (36). The water/salt-soluble extracts were sterilized by filtration through 0.22-μm membrane filters (Millipore Corporation, Bedford, MA) and added (30% [vol/vol] final concentration) to sterilized PDA. After mixing of the samples, aliquots of 15 ml were poured into petri plates (90-mm diameter). Control plates contained PDA alone. The assay was carried out by placing 3-mm-diameter plugs of growing mycelia onto the center of petri dishes containing the culture medium. Plates were incubated aerobically at 25°C. Three replicates were run simultaneously. The radial growth of mycelia (colony diameter, in mm) on all plates was measured 8 days after inoculation. Each datum point is the mean for at least four measurements of a growing colony. The percent growth inhibition was calculated from the mean values as follows: percent inhibition = [(mycelial growth under control conditions − mycelial growth in the presence of water/salt-soluble extract)/mycelial growth under control conditions] × 100.
The effect of water/salt-soluble extracts on the germination of conidia was also determined (24). After growth for 7 days on PDA plates, conidia of P. roqueforti DPPMAF1 were harvested in sterile water containing 0.05% (vol/vol) Tween 80. Counts of the conidia in the suspension were carried out using a Petroff-Hausser counting chamber. A fixed number of ca. 106 to 107 conidia/ml was added to 5 ml of the mixture of WFH containing 30% (vol/vol) water/salt-soluble extract. The mixtures were incubated in 60-mm petri dishes for 24 h at 25°C under stirring conditions. WFH alone and WFH supplemented with 0.3% (wt/vol) calcium propionate were used as the controls. To determine the percentage of germinated conidia (length/width ratio of ≥2), slides of the suspension were examined under a microscope (magnification, ×40) at 4-h intervals. Three separate replicates of at least 100 conidia were used for each assay.
To determine the MIC, samples were serially diluted in WFH. The mixture was poured into 60-mm petri dishes, inoculated with a suspension of conidia (106 to 107 conidia/ml as the final density), and incubated for 12 h at 25°C under stirring conditions. After 12 and 24 h, slides of the suspension were examined under the microscope and the germinated spores were counted as described above. The MIC was defined as the lowest concentration of water/salt-soluble extracts, related fractions, or ethanol/ethyl acetate aqueous solutions that inhibited fungal growth at 25°C for 12 h. All assays for antifungal activity were carried out at least in triplicate.
Proteolysis and heat stability of antifungal compounds.
Water/salt-soluble extracts were treated with trypsin (EC 3.4.21.4; Sigma Aldrich Co.) as described by Atanassova et al. (2). Heat stability was determined by heating extracts at 100°C for 5 min. After treatments, the residual activity was determined by agar diffusion assay.
Purification of antifungal compounds.
Water/salt-soluble extracts were fractionated by ultrafiltration (Ultrafree-MC centrifugal filter units; Millipore) using three different membrane sizes (50-, 30-, and 10-kDa cutoffs). Aliquots of 400 μl were centrifuged at 10,000 × g for 60 min. After ultrafiltration, fractions were used for agar diffusion assay.
Ethanol and ethyl acetate were detected by high-performance liquid chromatography (HPLC), using an Äkta Purifier system (GE Healthcare) equipped with an Aminex HPX-87H column (ion-exclusion column; Bio-Rad), a UV detector operating at 210 nm, and a Perkin Elmer 200a refractive index detector operating at 32°C. Elution was done at 60°C, with a flow rate of 0.6 ml/min, using 10 mM H2SO4 as the mobile phase (40).
Samples containing peptides were partially purified by reversed-phase fast-performance liquid chromatography (RP-FPLC), using a Resource RPC column and an Äkta FPLC instrument with a UV detector operating at 214 nm (GE Healthcare BioSciences AB, Uppsala, Sweden). Aliquots containing 0.875 mg/ml of peptides were added to 0.05% (vol/vol) trifluoroacetic acid (TFA) and centrifuged at 10,000 × g for 10 min. The supernatant was filtered through a 0.22-μm-pore-size filter and loaded onto the column. Gradient elution was performed at a flow rate of 1 ml/min, using a mobile phase composed of water and acetonitrile (CH3CN) containing 0.05% TFA. The CH3CN concentration was increased linearly from 5 to 46% between 16 and 62 min and from 46 to 100% between 62 and 72 min. Solvents were removed from collected fractions by freeze-drying. The fractions were redissolved in sterile water and subjected to agar diffusion assay. The freeze-dried preparation of the fraction with antifungal activity was used for identification.
A mixture of standard organic acids (lactic, acetic, caproic, propionic, butyric, n-valeric, phenyl lactic, and formic acids) with presumptive antifungal activity that might be synthesized by lactic acid bacteria during sourdough fermentation (10) was analyzed by RP-FPLC under the same conditions.
Identification and synthesis of antifungal peptides.
Identification of peptides was carried out by nano-liquid chromatography-electrospray ionization-tandem mass spectrometry (nano-LC-ESI-MS/MS), using a Finningan LCQ Deca XP Max ion trap mass spectrometer (ThermoElectron) through the nano-ESI interface. MS spectra were automatically taken by Xcalibur software (ThermoElectron) in positive ion mode according to the manufacturer's instructions. MS/MS spectra were processed using the software BioWorks 3.2 (ThermoElectron), generating peak lists suitable for database searches. Peptides were identified using MS/MS ion searches of the Mascot search engine (Matrix Science, London, England) and the NCBI nr protein database (National Center for Biotechnology Information, Bethesda, MD). For identification of peptides, the following parameters were considered: enzyme, “none”; instrument type, “ESI-trap”; peptide mass tolerance, ±0.1%; and fragment mass tolerance, ±0.5 Da. Peptide identification results were subjected to manual evaluation as described by Chen et al. (8), and the validated peptide sequences explained all the major peaks in the MS/MS spectrum. Identified peptides were chemically synthesized by NeoSystem Laboratoire (Strasbourg, France). The purity of the synthesized peptides was higher than 95%, as determined by high-performance liquid chromatography analysis and certified by the manufacturer.
Bread making.
Breads with a dough yield of 160 were manufactured at the pilot plant of the Department of Biologia e Chimica Agro-Forestale ed Ambientale, University of Bari, Italy. The formulas for bread making are reported in Table 1. Preliminarily, the formula for SB1A7 was used to make breads with sourdoughs fermented by L. rossiae 3D, L. plantarum 1A7, L. brevis AM7 or 5S, or L. fructivorans DSM20203. A continuous high-speed mixer (60 × g; dough mixing time, 5 min) was used to prepare the doughs. Fermentation was allowed at 30°C for 2.5 h, and breads were baked at 220°C for 40 min (Combo 3 oven; Zucchelli, Verona, Italy). For each bread, 2 slices were cut. The size of the slice was 12 cm (height) by 1.5 cm (width). One slice was inoculated by nebulization with a suspension of 102 conidia/ml of P. roqueforti DPPMAF1, and the other slice was not inoculated. Slices were packed in polyethylene bags to maintain constant moisture and then incubated at room temperature for 28 days. Moisture was determined according to the standard American Association of Cereal Chemistry (AACC) method (1).
Table 1.
Formulas for bread making
| Ingredient | Amt in breadd |
||||
|---|---|---|---|---|---|
| WB | WBcp | SB1A7 | D1695 | SB1A7-1695 | |
| Wheat flour (g) | 250 | 250 | 175 | 175 | 175 |
| Tap water (ml) | 150 | 150 | 105 | 105 | 105 |
| S1A7a (g) | 120 | ||||
| D1695b (g) | 120 | ||||
| S1A7-1695c (g) | 120 | ||||
| Calcium propionate (g) | 1.2 | ||||
| Baker's yeast (g) | 8 | 8 | 8 | 8 | 8 |
Sourdough containing 62.5% flour and 37.5% water (dough yield of 160) and inoculated with Lactobacillus plantarum 1A7 (108 CFU/g).
Dough containing 62.5% flour and 37.5% water (dough yield of 160) and inoculated with Wickerhamomyces anomalus LCF1695 (106 CFU/g).
Sourdough containing 62.5% flour, 37.5% water (dough yield of 160) and inoculated with Lactobacillus plantarum 1A7 (108 CFU/g) and Wickerhamomyces anomalus LCF1695 (106 CFU/g).
The dough yield of all breads was 160.
Bread characterization.
The pH values were determined online by use of a pH meter (model 507; Crison, Milan, Italy) with a food penetration probe.
Instrumental texture profile analysis (TPA) was carried out with a TA.XT2i texture analyzer, using a 35-mm flat-end aluminum compression disk (probe P/35). The selected settings were as follows: test speed of 1 mm/s, 30% deformation of the sample, and one compression cycle. TPA was carried out using Texture Export Exceed software (39). Specific volume was measured by the TA.XT2i system. The following textural parameters were obtained by the texturometer software: hardness (maximum peak force), fracturability (the first significant peak force during the probe compression of the bread), and resilience (area during the withdrawal of the penetration divided by the area of the first penetration). The crumb grain of breads was evaluated after 24 h of storage, using image analysis technology. Images of the sliced breads were captured using an image scanner (Amersham Pharmacia Biotech, Uppsala, Sweden). Images were scanned full-scale at 300 dots per inch and analyzed in gray scale (0 to 255). Image analysis was performed using the UTHSCSA ImageTool program (version 2.0; University of Texas Health Science Centre, San Antonio, TX [available by anonymous FTP from ftp://maxrad6.uthscsa.edu]). A threshold method was used for differentiating gas cells and noncells. The threshold was determined according to the method of Crowley et al. (13). Analysis was carried out on two subimages of 500 by 500 pixels (field of view) selected from within the bread slice. Two slices were analyzed per treatment. The crumb cell features recovered were number, area, perimeter, and ratio of gas cell area to total area.
Color was measured at three different positions of the bread surface by use of a Minolta CR-10 camera (2). The L*a*b* color space analysis method was used, where L* represents lightness (white-black) and a* and b* represent the chromaticity coordinates (red-green and yellow-blue, respectively). Results were reported in the form of a color difference, dE*ab, as follows:
where dL, da, and db are the differences for L, a, and b values between the sample and the reference (a white ceramic plate having an L value of 93.4, an a value of −1.8, and a b value of 4.4).
Sensory analysis of breads was carried out by 10 panelists (5 male and 5 female; mean age, 35 years; age range, 18 to 54 years) according to the method described by Haglund et al. (21). Elasticity, color, acid taste, acid flavor, sweetness, dryness, and taste were the considered sensory attributes for breads (21), using a scale of 0 to 10, with 10 being the highest score. The sensory attributes were discussed with the assessors during the introductory sensory training sessions. Samples were served in random order and evaluated in two replicates by all panelists. In preparation for the sensory evaluation, the loaves were thawed at room temperature for 5 to 6 h and then cut into 1.5-cm-thick slices. Slices were cut into 4 pieces, and each assessor received 2 pieces per sample.
Statistical analysis.
Data were subjected to one-way analysis of variance (ANOVA). Pairwise comparison of treatment means was achieved by Tukey's procedure with a significance level of P values of <0.05, using the statistical software Statistica 7.0 for Windows.
RESULTS
Selection of lactic acid bacteria and yeasts.
As shown by agar diffusion assay, only 5 of the 40 water/salt-soluble extracts from sourdoughs had marked antifungal activity toward the indicator organism P. roqueforti DPPMAF1. These sourdoughs were fermented by L. rossiae 5A, L. plantarum 1A7, L. brevis AM7 or 5S, or L. fructivorans DSM20203. These sourdoughs were used for bread making. After 7 days of storage, only the slices of bread manufactured with the sourdough fermented by L. plantarum 1A7 (S1A7) did not show fungal contamination by P. roqueforti DPPMAF1, as determined by a visual inspection.
Regarding yeasts, the water/salt-soluble extract from the dough fermented by W. anomalus LCF1695 (D1695) showed the most inhibition of the indicator organism P. roqueforti DPPMAF1 (ca. 3 mm of clear zone near the rim of the colony).
Doughs fermented by L. plantarum 1A7 and W. anomalus LCF1695 were selected and used for further characterization.
Identification of antifungal compounds.
The buffered water/salt-soluble extract of S1A7 had a pH of 7.34 ± 0.03. The concentrations of proteins and peptides were 2.38 ± 0.21 and 1.92 ± 0.13 mg/ml, respectively. As shown by agar diffusion assays, the antifungal activity was almost completely lost by trypsin digestion, while it was unaffected by heating at 100°C for 5 min. Preliminarily, the water/salt-soluble extract of S1A7 was fractionated by ultrafiltration. Inhibitory activity toward P. roqueforti DPPMAF1 was found in all three fractions (cutoffs of 50, 30, and 10 kDa). As a consequence, further characterization was carried out only with the fraction showing a molecular mass of <10 kDa. This fraction was subjected to partial purification through RP-FPLC, and 37 fractions were separated (the concentrations of peptides varied from 0.30 ± 0.02 to 5.61 ± 0.04 mg/ml). Although weak antifungal activity was found in fractions 2, 3, and 21 (0.25 ± 0.01, 0.47 ± 0.02, and 2.04 ± 0.02 mg of peptide/ml, respectively), the most inhibition was found for fractions 12 and 13 (3.25 ± 0.03 and 4.21 ± 0.01 mg of peptide/ml, respectively). As expected, the mixture of organic acids used as the standard eluted in the hydrophilic zone of the acetonitrile gradient, which corresponded to fractions 1, 2, and 3. Therefore, the inhibitory activity of fractions 12, 13, and 21 was presumptively related to compounds different from organic acids.
The peptides contained in fractions 12, 13, and 21 were identified by nano-LC-ESI-MS/MS followed by MS/MS ion searches with the Mascot search engine. Nine peptides of 14 to 55 amino acid residues were identified (Table 2). Peptides PPDVLTKLTAVPAAQQLDEADGHPR, SAFEFADEHKGAYS, AAIIFGSIFWNVGMKR, and ALGFEMTPEQIHQMI were identified in fraction 12. These peptides had experimental molecular masses of 2,638.37, 1,557.67, 1,808.98, and 1,743.83 Da, respectively. Except for AAIIFGSIFWNVGMKR, which had a positive charge (+2) and a total hydrophobic ratio of 62%, the other peptides had a negative charge. SAFEFADEHKGAYS was also identified in fraction 13, which contained two other peptides, with molecular masses of 5,678.71 and 1,785.86 Da, a net negative charge, and total hydrophobic ratios of 30 and 35%. The peptides EGTYLDYIQNNGKTLGAEDSTNEFGWDNK, WFVELTGIPVTTTLMGLGNFPSDDPLSLRMLGMHGTVYANYAVDK, and DEEIMLDITTCAMAFRLLR were identified in fraction 21. They had total net charges of −4, −1, and −2 and hydrophobic ratios of 20, 42, and 57%, respectively. By searches of the NCBI nr database, all peptides were reported as encrypted in sequences of Oryza sativa proteins. NCBI accession numbers of source proteins are listed in Table 2.
Table 2.
Sequences of peptides identified in partially purified fractions of water/salt-soluble extract of sourdough fermented with Lactobacillus plantarum 1A7
| Fraction | Sequencea | Score | Charge | Calculated mass (Da) | Expected mass (Da) | Delta | Source protein (NCBI accession no.) |
|---|---|---|---|---|---|---|---|
| 12 | PPDVLTKLTAVPAAQQLDEADGHPR | 24 | 2 | 2,638.3715 | 2,638.2228 | −0.1487 | KNOS8_ORYSJ, homeobox protein knotted-1-like 8 (Q10ED2) |
| SAFEFADEHKGAYS | 17 | 2 | 1,557.6736 | 1,558.0624 | 0.3888 | GUN23_ORYSJ, endoglucanase 23 (Q69NF5) | |
| AAIIFGSIFWNVGMKR | 17 | 2 | 1,808.9760 | 1,808.4154 | −0.5606 | PDR13_ORYSJ, pleiotropic drug resistance protein 13 (Q8S628) | |
| ALGFEMTPEQIHQMI | 16 | 2 | 1,743.8324 | 1,744.5257 | 0.6953 | CML8_ORYSJ, probable calcium-binding protein CML8 (Q338P8) | |
| 13 | IGRDVQNQSLFSPQVDSSSLLYNMVPNLTSNVSDGNLSTIPSGSTYLQNAMYG | 18 | 3 | 5,678.7145 | 5,679.5927 | 0.8782 | ARFL_ORYSI, auxin response factor 12 (Q258Y5) |
| SAFEFADEHKGAYS | 17 | 2 | 1,557.6736 | 1,558.0624 | 0.3888 | GUN23_ORYSJ, endoglucanase 23 (Q69NF5) | |
| AEGEVILEDVQPSSVQS | 14 | 2 | 1,785.8632 | 1,785.2057 | −0.6576 | COPD1_ORYSJ, coatomer subunit delta-1 OS (Oryza sativa subsp. japonica) (Q0DJA0) | |
| 21 | EGTYLDYIQNNGKTLGAEDSTNEFGWDNK | 31 | 3 | 3,278.4640 | 3,278.8756 | 0.4117 | GUN23_ORYSJ, endoglucanase 23 (Q69NF5) |
| DEEIMLDITTCAMAFRLLR | 13 | 2 | 2,240.1003 | 2,239.8321 | −0.2682 | KS1_ORYSJ, ent-kaur-16-ene synthase, chloroplastic (Q0JA82) | |
| WFVELTGIPVTTTLMGLGNFPSDDPLSLRMLGMHGTVYANYAVDK | 13 | 3 | 4,926.4435 | 4,925.9840 | −0.4595 | ILVB1_ORYSJ, acetolactate synthase 1, chloroplastic (Q6K2E8) |
The single-letter amino acid code is used.
Partially purified fractions 12, 13, and 21 were freeze-dried, redissolved in WFH to have a range of concentrations of 0.5 to 20.0 mg of peptide/ml, and used to determine the MIC based on conidial germination. No germination of P. roqueforti DPPMAF1 conidia was found at concentrations of 3.9, 4.5, and 9.6 mg peptide/ml for fractions 12, 13, and 21, respectively. Peptides identified in the most active fractions (12 and 13) were chemically synthesized and used to determine the MIC. When used singly, SAFEFADEHKGAYS, AAIIFGSIFWNVGMKR, AEGEVILEDVQPSSVQS, and PPDVLTKLTAVPAAQQLDEADGHPR confirmed the inhibitory activity. The range of MICs varied from 2.5 to 11.2 mg/ml. The other synthesized peptide also did not show appreciable inhibition at the highest concentration assayed. Among all possible combinations, the lowest MIC (ca. 2.05 mg of peptides/ml) was found when SAFEFADEHKGAYS and AAIIFGSIFWNVGMKR were used in a mixture.
The antifungal activity of the water/salt-soluble extract of D1695 (pH 7.82 ± 0.02) persisted after trypsin digestion or after heating at 100°C for 5 min. Protein derivatives were therefore excluded as the source of inhibitory activity. HPLC analysis of the water/salt-soluble extract of D1695 showed concentrations of ethanol and ethyl acetate of 1.48 (35.1 mM) and 6.50 (73.8 mM) mg/ml, respectively. Various aqueous solutions, containing ethanol and ethyl acetate at a molar ratio of 35:74, were used to determine the MIC. No germination of P. roqueforti DPPMAF1 conidia was found at concentrations of ethanol and ethyl acetate of 1.69 (36.6 mM) and 6.81 (77.0 mM) mg/ml, respectively.
Characterization of antifungal activity.
The dough fermented with W. anomalus LCF1695 (D1695) and the sourdoughs started with L. plantarum 1A7 (S1A7) and with strains 1A7 and LCF1695 (S1A7-1695) were characterized. After fermentation, L. plantarum 1A7 was found at cell densities of 6.5 × 109 and 3.4 × 109 CFU/g in S1A7 and S1A7-1695, respectively. W. anomalus LCF1695 was found at cell densities of 6.2 × 107 and 2.2 × 107 CFU/g in D1695 and S1A7-1695, respectively. After 16 h of fermentation at 30°C, the pH values were 3.90 ± 0.02, 5.05 ± 0.01, and 3.79 ± 0.02 for S1A7, D1695, and S1A7-1695, respectively. As shown by HPLC analysis, the concentrations of ethanol and ethyl acetate in the water/salt-soluble extract of S1A7-1695 were 1.65 (35.8 mM) and 5.07 (57.5 mM) mg/ml, respectively.
After incubation for 12 h at 25°C on WFH, the mean percentage of spore germination of P. roqueforti DPPMAF1 was 16.9% ± 0.9% (Fig. 1). Incubation in the presence of the water/salt-soluble extracts of S1A7, D1695, and S1A7-1695 caused significant (P < 0.05) decreases of conidial germination, to values below 9.0%. In particular, the highest inhibitory activity was obtained with the water/salt-soluble extract of D1695 (7.0% ± 0.1%). The inhibitory activity of 0.3% (wt/vol) calcium propionate was slightly higher (7.8% ± 0.3%). After 24 h, the percentage of conidial germination in WFH reached 90% ± 0.7%. The inhibitory activity of 0.3% (wt/vol) calcium propionate (26% ± 0.3%) was lower than those found in the water/salt-soluble extracts of the selected dough and sourdoughs. The lowest value for conidial germination was still found for D1695 (9.7% ± 0.5%).
Fig. 1.
Conidial germination of Penicillium roqueforti DPPMAF1 incubated in WFH for 12 and 24 h at 25°C under different conditions. Treatments were as follows: Ct, WFH alone (control); CP, WFH supplemented with 0.3% (wt/vol) calcium propionate; S1A7, WFH supplemented with 30% water/salt-soluble extract of sourdough fermented with Lactobacillus plantarum 1A7; D1695, WFH supplemented with 30% water/salt-soluble extract of dough fermented with Wickerhamomyces anomalus LCF1695; and S1A7-1695, WFH supplemented with 30% water/salt-soluble extract of sourdough fermented with L. plantarum 1A7 and W. anomalus LCF1695. Data are the means for three independent experiments, and the values within series marked with different letters are statistically significantly different (P < 0.05).
The activities of the water/salt-soluble extracts of S1A7, D1695, and S1A7-1695 were assayed against several fungi isolated from bakeries (Table 3). The growth inhibition was measured on PDA after 8 days of incubation at 25°C (Fig. 2). All fungi were variously affected. Regarding S1A7, the highest percentages of inhibition were found for A. parasiticus CBS 971.97 (58.2% ± 0.7%) and P. polonicum CBS 112490 (56.6% ± 1.5%). Weak antifungal activity was found against P. albocoremium CBS 109582, A. versicolor CBS 117286, E. herbariorum CBS 117336, and P. bialowiezense CBS 110102. Except for assays with P. bialowiezense CBS 110102 and Eurotium rubrum CBS 150.92, the activities of the water/salt-soluble extract of D1695 were significantly (P < 0.05) higher than those of S1A7. The highest values of inhibition were found for P. polonicum CBS 112490 (61.5% ± 1.1%), A. parasiticus CBS 971.97 (58.2% ± 0.9%), and P. polonicum CBS 112490 (56.6% ± 0.3%). P. chrysogenum CBS 111214, P. albocoremium CBS 109582, and A. versicolor CBS 117286 were significantly (P < 0.05) more inhibited by the water/salt-soluble extract of S1A7-1695 than by those of S1A7 and D1695, whereas the growth inhibition was intermediate for the other fungi tested (Table 3).
Table 3.
Inhibitory spectra of water/salt-soluble extracts of sourdoughs or dough fermented with Lactobacillus plantarum 1A7 (S1A7), Wickerhamomyces anomalus LCF1695 (D1695), or a combination of strains 1A7 and 1695 (S1A7-1695) (30% [vol/vol] on PDA)
| Fungal strain | Source, country of isolation | Inhibitory activity (%)a |
||
|---|---|---|---|---|
| S1A7 | D1695 | S1A7-1695 | ||
| Penicillium roqueforti DPPMAF1 | Bread, Italy | 42.6 ± 1.2b | 47.2 ± 0.8a | 45.6 ± 1.0a |
| Penicillium polonicum CBS 112490 | Bread, Italy | 56.6 ± 1.5c | 61.5 ± 1.1a | 58.5 ± 0.5b |
| Penicillium chrysogenum CBS 111214 | Bread, England | 23.6 ± 0.4c | 26.3 ± 0.5b | 34.2 ± 1.1a |
| Penicillium paneum CBS 101032 | Rye bread, Denmark | 43.9 ± 0.6c | 59.7 ± 0.5a | 47.6 ± 0.9b |
| Penicillium albocoremium CBS 109582 | Cake factory, Denmark | 4.3 ± 0.3c | 10.8 ± 0.4b | 13.9 ± 0.5a |
| Penicillium chermesinum CBS 117279 | Bakery plant, Netherlands | 43.6 ± 0.7b | 56.3 ± 1.1a | 43.6 ± 0.9b |
| Eurotium herbariorum CBS 117336 | Chocolate cake, Netherlands | 7.7 ± 0.5c | 46.1 ± 0.5a | 23.2 ± 0.6b |
| Eurotium rubrum CBS 150.92 | Cake | 18.4 ± 0.3a | 10.5 ± 0.6c | 15.3 ± 0.6b |
| Aspergillus parasiticus CBS 971.97 | Indian sweets, India | 58.2 ± 0.7 | 58.5 ± 0.3 | 58.3 ± 0.9 |
| Penicillium carneum CBS 112297 | Rye bread, Denmark | 45.6 ± 0.6c | 59.6 ± 0.8a | 48.3 ± 0.9b |
| Aspergillus versicolor CBS 117286 | Wall in bakery, Netherlands | 5.3 ± 0.4c | 12.3 ± 0.5b | 14.8 ± 0.5a |
| Penicillum bialowiezense CBS | Bread, Italy | 9.5 ± 0.6a | 4.7 ± 0.6b | 8.7 ± 0.4a |
Inhibitory activity was determined by hyphal radial growth rates of fungi (36) after 8 days of incubation at 25°C. The percentage of growth inhibition was calculated from mean values as follows: % inhibition = [(mycelial growth under control conditions − mycelial growth in the presence of water/salt-soluble extract)/mycelial growth under control conditions] × 100. Data are means and standard deviations for at least four measurements of a growing colony. Means within a row with different superscript letters are significantly different (P < 0.05).
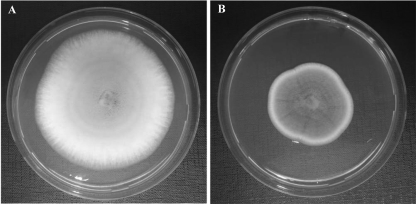
Fig. 2.
Antifungal activity of water/salt-soluble extract of dough fermented with Wickerhamomyces anomalus LCF1695 (D1695), as determined by hyphal radial growth inhibition (44) after 8 days of incubation at 25°C. (A) PDA alone (control); (B) PDA containing water/salt-soluble extract of D1695 (30% [vol/vol]).
Bread making.
Five breads were manufactured at pilot plant scale. The pH values were 5.45 ± 0.08, 5.44 ± 0.05, 4.82 ± 0.05, 5.24 ± 0.05, and 4.83 ± 0.02 for wheat flour bread (WB), wheat flour bread containing calcium propionate (WBcp), SB1A7, B1695, and SB1A7-1695, respectively (Table 4). The specific volumes of breads for which sourdough fermentation was used were always the highest (2.25 ± 0.04 and 2.40 ± 0.04 g/cm3 for SB1A7 and SB1A7-1695, respectively). Overall, for all parameters considered, no significant (P > 0.05) differences were found between WB and WBcp. The concentrations of ethanol and ethyl acetate were determined in the water/salt-soluble extracts of B1695 and SB1A7-1695. A slightly larger amount of ethyl acetate was found in the water/salt-soluble extract of SB1A7-1695 (20.4 mM versus 19.5 mM), while both extracts contained ca. 21.7 mM ethanol.
Table 4.
Characterization and texture profile analysis of wheat flour bread (WB), wheat flour bread containing calcium propionate (WBcp), sourdough bread fermented with Lactobacillus plantarum 1A7 (SB1A7), bread fermented with Wickerhamomyces anomalus LCF1695 (B1695), and sourdough bread fermented with strains 1A7 and LCF1695 (SB1A7-1695)
| Parameter | Valuea |
||||
|---|---|---|---|---|---|
| WB | WBcp | SB1A7 | B1695 | SB1A7-1695 | |
| pH | 5.45 ± 0.08c | 5.44 ± 0.05c | 4.82 ± 0.05a | 5.24 ± 0.05b | 4.83 ± 0.02a |
| Moisture | 31.2 ± 0.3 | 31.4 ± 0.1 | 31.0 ± 0.2 | 31.4 ± 0.1 | 31.0 ± 0.2 |
| TPA | |||||
| Sp vol (g/cm3) | 1.92 ± 0.02b | 1.90 ± 0.03b | 2.25 ± 0.04c | 1.84 ± 0.03a | 2.40 ± 0.04d |
| Density (cm3/g) | 0.52 ± 0.02c | 0.53 ± 0.02c | 0.45 ± 0.02b | 0.56 ± 0.02d | 0.39 ± 0.02a |
| Hardness (g) | 2,792 ± 12c | 2,799 ± 22c | 2,578 ± 25b | 2,889 ± 22d | 2,378 ± 25a |
| Resilience | 0.65 ± 0.02c | 0.64 ± 0.03c | 0.36 ± 0.01b | 0.67 ± 0.03d | 0.32 ± 0.01a |
| Fracturability (J) | 39,897 ± 28c | 39,901 ± 24c | 35,702 ± 19b | 41,101 ± 24d | 35,002 ± 19a |
| Image analysis | |||||
| Black pixel area (%) | 39.23 ± 0.1b | 38.98 ± 0.1b | 42.28 ± 0.20c | 34.76 ± 0.15a | 44.18 ± 0.20d |
| No. of cells detected | 118 ± 3a | 120 ± 3a | 188 ± 5c | 135 ± 4b | 231 ± 5d |
| Mean area (mm2) | 3.59 ± 0.02c | 3.58 ± 0.02c | 2.16 ± 0.02a | 3.20 ± 0.03b | 2.22 ± 0.02a |
| Mean perimeter (mm) | 1.52 ± 0.01c | 1.49 ± 0.01c | 1.25 ± 0.01a | 1.35 ± 0.02b | 1.25 ± 0.03a |
| Color analysis | |||||
| L | 61.27 ± 0.10c | 61.09 ± 0.10c | 58.93 ± 0.08b | 61.31 ± 0.10c | 56.73 ± 0.08a |
| a | 11.66 ± 0.02b | 11.62 ± 0.02b | 15.33 ± 0.02c | 11.55 ± 0.02a | 15.35 ± 0.02c |
| b | 33.8 ± 0.02b | 33.6 ± 0.01b | 31.03 ± 0.02a | 33.2 ± 0.03b | 31.05 ± 0.02a |
| dE | 45.58a | 45.57a | 46.81b | 45.14a | 48.47c |
Triplicate measurements were made for each bread, and means ± standard deviations are reported. Means within a row with different superscript letters are significantly different (P < 0.05). The ingredients used for bread making are listed in Materials and Methods.
The value of hardness, corresponding to the peak force of the first compression of the product, was the highest for B1695 (2,889 ± 22 g), and it differed significantly (P < 0.05) between SB1A7, SB1A7-1695, and WB (Table 4). Resilience indicates how well a product fights to regain its original position. The lowest value for resilience was found for SB1A7-1695 (0.32 ± 0.01), followed by SB1A7 (0.36 ± 0.01). The highest value (0.67 ± 0.03) was found for B1695. The fracturability point corresponds to the first significant peak force during compression of the bread. In this case, the highest value was also found for B1695 (41,101 ± 24 J). Significant (P < 0.05) differences were also found between SB1A7 and SB1A7-1695 (35,702 ± 19 and 35,002 ± 19 J, respectively).
The crumb grain of the breads was determined by image analysis technology. Digital images were preprocessed to detect crumb cell total area by a binary conversion (black/white pixels) (Table 4). The cell total area (corresponding to the black pixel total area) was highest for SB1A7-1695 (44.18% ± 0.20%), followed by SB1A7 (42.28% ± 0.20%). The cell total area for B1695 was lower than that found for WB (34.76% ± 0.15% and 39.23% ± 0.1%, respectively). Crumb cell detection with bread slice portions showed some differences in the numbers of gas cells. The number of cells for SB1A7-1695 (231 ± 5) was the highest, followed by SB1A7 (188 ± 5). The mean areas and mean perimeters of cells for SB1A7-1695 (2.22 ± 0.02 mm2 and 1.25 ± 0.01 mm, respectively) and SB1A7 (2.16 ± 0.02 mm2 and 1.25 ± 0.01 mm, respectively) differed markedly (P < 0.05) from those found for B1695 and WB.
The crust showed a decrease in lightness (L) from 61.31 ± 0.10 to 56.73 ± 0.08, which corresponded to B1695 and SB1A7-1695, respectively (Table 4). The color difference (dE), calculated based on the chromaticity coordinates L, a, and b, was not significantly different for WB, WBcp, or B1695. Significant (P < 0.05) differences were found for SB1A7 and SB1A7-1695 (dE values of 46.81 and 48.47, respectively).
A few hours after bread baking, sensory analysis was carried out (Table 5). SB1A7 and SB1A7-1695 received high scores for elasticity. In addition, the same two breads received the highest scores for acid taste and flavor. The scores for color were similar for WB, WBcp, and B1695. They differed significantly (P < 0.05) between SB1A7 (6.6 ± 0.6) and SB1A7-1695 (7.6 ± 0.6). Sweetness scores were slightly higher for WB and WBcp. As previously reported (11, 38), the scores for dryness (how much the bread crumbles in the mouth) for sourdough-fermented SB1A7 and SB1A7-1695 were lower than that for baker's yeast bread (Table 5). The overall perception of taste was the highest for SB1A7-1695 (7.7 ± 0.5), followed by SB1A7, B1695, and WB and WBcp.
Table 5.
Sensory analysis of WB, WBcp, SB1A7, B1695, and SB1A7-1695
| Sensory attribute | Scorea |
||||
|---|---|---|---|---|---|
| WB | WBcp | SB1A7 | B1695 | SB1A7-1695 | |
| Elasticity | 7.5 ± 0.4b | 7.5 ± 0.4b | 7.5 ± 0.6b | 7.0 ± 0.6a | 7.5 ± 0.6b |
| Color | 4.5 ± 0.5a | 4.5 ± 0.8a | 6.6 ± 0.6b | 4.6 ± 0.6a | 7.6 ± 0.6c |
| Acid taste | 2.3 ± 0.1a | 2.5 ± 0.5a | 6.5 ± 0.6c | 4.4 ± 0.6b | 7.0 ± 0.6d |
| Acid flavor | 2.5 ± 0.5a | 2.3 ± 0.1a | 6.0 ± 0.8c | 3.8 ± 0.8b | 6.3 ± 0.8c |
| Sweetness | 6.2 ± 0.5b | 6.0 ± 0.6b | 5.4 ± 0.3a | 5.4 ± 0.3a | 5.4 ± 0.3a |
| Dryness | 5.8 ± 0.2c | 5.6 ± 0.2c | 3.3 ± 0.5a | 4.0 ± 0.2b | 3.5 ± 0.3a |
| Taste | 3.8 ± 0.3a | 4.0 ± 0.6b | 7.0 ± 0.5d | 6.1 ± 0.5c | 7.7 ± 0.5d |
Sensory attributes were scored by using a scale of 0 to 10. Data are means ± standard deviations for fermentations analyzed in duplicate. Means within a row with different superscript letters are significantly different (P < 0.05). The ingredients used for bread making are listed in Materials and Methods.
After the bread was baked, 2 slices of each bread were cut and inoculated with a suspension of P. roqueforti DPPMAF1 (102 conidia/ml). Slices were packed with polyethylene film and stored at room temperature. The fungal contamination of the bread slices was observed throughout 28 days (Table 6). The moisture of all slices was in the range of 30.5 to 31.4% during the 28 days.
Table 6.
Fungal contamination of slices of WB, WBcp, SB1A7, B1695, and SB1A7-1695 packed in polyethylene bags and incubated at room temperature for 28 days
| Day | Contaminationa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inoculated slices |
Noninoculated slices |
|||||||||
| WB | WBcp | SB1A7 | B1695 | SB1A7-1695 | WB | WBcp | SB1A7 | B1695 | SB1A7-1695 | |
| 7 | ++ | − | − | − | − | + | − | − | − | − |
| 14 | +++ | + | + | − | − | ++ | − | − | − | − |
| 21 | ++++ | ++ | ++ | + | +/− | +++ | + | + | +/− | − |
| 28 | ++++ | +++ | ++ | ++ | +/− | ++++ | ++ | ++ | + | − |
Contamination was scored as follows: −, 0% contamination of the slice surface; +/−, 10% contamination; +, 20% contamination; ++, 40% contamination; +++, 80% contamination; ++++, 100% contamination. For each bread, a slice was inoculated by nebulization with a suspension of 102 conidia/ml of Penicillium roqueforti DPPMAF1, and the other slice was not inoculated. Fermentations were carried out in triplicate, and each bread was analyzed twice.
Among the inoculated slices, only the WB slices were colonized by P. roqueforti DPPMAF1 after 7 days of storage. The growth of P. roqueforti DPPMAF1 was observed in WBcp and SB1A7 after 14 days of storage. At 28 days, only SB1A7-1695 showed weak fungal growth, while in all other slices the fungal contamination was higher than 20% (Table 6). Among the noninoculated slices, weak fungal contamination appeared in WB at 7 days, whereas no fungal growth was evident in the other cases. The fungal growth was also visible at 21 days in the slices of WBcp and SB1A7, while it was lower for B1695 (Table 6). No fungal contamination was noticeable for SB1A7-1695 at 28 days of storage (Table 6). After 28 days, the moisture of the slices gradually decreased (to values below 29.0%), indicating that no growth of molds was probably due to the decrease of the water activity.
DISCUSSION
Yearly, the growth of fungi destroys large amounts of vegetables, fruits, cereal grains, ingredients, foods, and feed (35). Beyond economic losses, the growth of fungi in foods causes a decrease of the nutritional value and the synthesis of allergenic spores and hazardous mycotoxins (23). Traditionally, fungicides and chemical preservatives have been used to prevent fungal growth, but concerns about environmental pollution and consumer health, along with problems of microbial resistance, favor the demand for alternative methods of control (16).
Besides the synthesis of other inhibitory compounds, lactic acid bacteria may release, through proteolysis, encrypted antimicrobial peptides from food proteins (10, 37). The purification of the antifungal compounds contained in the water/salt-soluble extract of sourdough fermented by L. plantarum 1A7 allowed the identification of 9 novel antifungal peptides. Several of the identified peptides show homology with known antifungal peptide sequences (47). The sequence SAFEFADEHKGAYS was commonly found in two partially purified fractions. It is encrypted in the cereal enzyme endoglucanase 23 (NCBI accession no. Q69NF5). The antifungal activity of glucanases has already been shown by in vitro assays as well as by using transgenic plants (41). As shown in the Antimicrobial Peptide Database (47), using prediction and design tools, the AAIIFGSIFWNVGMKR peptide may have a helix conformation with at least 6 residues on the same hydrophobic surface. This allows us to hypothesize an antimicrobial activity due to interactions with microbial membranes. The sequence ALGFEMTPEQIHQMI has 36% homology with the antimicrobial peptide alpha-basrubrin, identified in Ceylon spinach seeds (46). The peptide EGTYLDYIQNNGKTLGAEDSTNEFGWDNK has 36% homology with the lantibiotic lacticin 3147 (24). Overall, the MICs of the partially purified fractions from the water/salt-soluble extract of the sourdough fermented with L. plantarum 1A7 were lower than 10 mg/ml. These concentrations compared well with those of similar proteinaceous substances which also showed antifungal activities (10, 37, 40). Furthermore, the MIC of the mixture of the two chemically synthesized peptides SAFEFADEHKGAYS and AAIIFGSIFWNVGMKR was 2.05 mg/ml. The synergistic activity of antimicrobial peptides has been reported previously (17).
Yeasts also have promising features for use as alternatives to chemical fungicides (7, 35). Yeasts do not produce mycotoxins or allergenic spores, the vast majority are not pathogenic to humans or other animals, and several species grow at low values for water activity and at low partial pressures of O2 (16, 34). Previously, other reports (3, 5, 16, 32) showed that W. anomalus (formerly known as Hansenula anomala or Pichia anomala) decreased the growth of P. roqueforti during storage of cereal grains. W. anomalus was the best among 60 different yeast species in the capacity to inhibit the growth of P. roqueforti in grain silo plants (16). W. anomalus decreased the accumulation of ochratoxin A during growth in coculture with Penicillium verrucosum (34). The water/salt-soluble extract of W. anomalus LCF1695 was not affected by either enzyme digestion or thermal treatment. This probably excluded an inhibitory activity related to protein killer toxins or cell wall-degrading enzymes, which are often synthesized by W. anomalus strains (45). Ethanol and ethyl acetate were also considered responsible for the antifungal activity of W. anomalus (4). In particular, ethyl acetate is considered the key inhibitory component (16), while a marginal but synergic role has been attributed to ethanol (16). Concentrations of 35.1 and 73.8 mM ethanol and ethyl acetate were found in the water/salt-soluble extract of the dough fermented with W. anomalus LCF1695. Ethyl acetate is the most significant ester synthesized by yeasts during fermentation (44). Compared to that in Saccharomyces cerevisiae or other yeasts, the synthesis of ethyl acetate by W. anomalus occurs via an inverse esterase from acetate rather than from acetyl-coenzyme A (acetyl-CoA) via ethanol acetyltransferase (30). Considerable levels of ethyl acetate were synthesized by W. anomalus during in vitro growth or simulated biocontrol in silos with airtight-stored grain (16, 30), but no reports previously showed its synthesis and antifungal effect during bread making.
With the aim of comparing antifungal activities and technological properties, three doughs or sourdoughs fermented by W. anomalus LCF1695, L. plantarum 1A7, and their combination were manufactured according to traditional sourdough technology. As already shown for calcium propionate at a concentration of 0.3% (wt/vol) (10, 23), a weak effect against conidial germination was found for the sourdough fermented with L. plantarum 1A7, which suggested a fungistatic activity (10). After 24 h, the germination of P. roqueforti DPPMAF1 decreased ca. 90% when it was treated with the dough fermented by W. anomalus LCF1695. In agreement, a marked decrease of the germination of several fungi was found in the presence of strains of W. anomalus synthesizing ethyl acetate (32). The water/salt-soluble extract of the dough fermented by W. anomalus LCF1695 showed a large spectrum of activity toward fungal species which are commonly isolated from contaminated baked goods. The inhibition was higher than 45% for 7 of the 12 fungi tested. The spectrum of activity was narrower and the activity was generally lower during treatment with the water/salt-soluble extract of the sourdough fermented with L. plantarum 1A7. As expected, the sourdough fermented with both strains had an intermediate antifungal activity. The synthesis of ethyl acetate by W. anomalus LCF1695 slightly decreased during fermentation with L. plantarum 1A7. Compared to antifungal peptides, a predominant role of ethyl acetate was hypothesized.
Despite W. anomalus being found commonly in artisan bakery sourdoughs (14), to the best of our knowledge no reports have previously considered its use as a starter for sourdough fermentation (15). The bread manufactured with the combination of L. plantarum 1A7 and W. anomalus LCF1695 showed the most volume and softness, and in a sensory analysis, it was appreciated for elasticity, color, and overall taste. Although the concentration of ethyl acetate decreased in part during bread making under pilot plant conditions, this bread showed a very prolonged shelf life. Fungal growth was delayed at least until 28 days of storage at room temperature under common storage conditions. The inhibitory activity was higher than that found for calcium propionate (0.3% [wt/wt]). This work demonstrates the use of the nonconventional yeast W. anomalus as a mixed starter for sourdough fermentation to extend the shelf life of baked goods while also keeping optimal taste and structure.
Footnotes
Published ahead of print on 25 March 2011.
REFERENCES
- 1. AACC 2003. Approved methods of the American Association of Cereal Chemistry, 10th ed. AACC, St. Paul, MN [Google Scholar]
- 2. Atanassova M., et al. 2003. Isolation and partial biochemical characterization of a proteinaceous anti-bacteria and anti-yeast compound produced by Lactobacillus paracasei subsp. paracasei strain M3. Int. J. Food Microbiol. 87:63–73 [DOI] [PubMed] [Google Scholar]
- 3. Björnberg A., Schnürer J. 1993. Inhibition of the growth of grain-storage molds in vitro by the yeast Pichia anomala (Hansen) Kurtzman. Can. J. Microbiol. 39:623–628 [Google Scholar]
- 4. Borling J. 2010. Feed improvement by energy efficient storage using Pichia anomala inoculated ensiled cereal grain. M.S. thesis. Uppsala BioCenter, Department of Microbiology, Faculty of Natural Resources and Agriculture Science, Swedish University of Agricultural Science, Uppsala, Sweden [Google Scholar]
- 5. Boysen M. E., Björneholm S., Schnürer J. 2000. Effect of the biocontrol yeast Pichia anomala on interactions between Penicillium roqueforti, Penicillium carneum, and Penicillium paneum in moist grain under restricted air supply. Postharvest Biol. Technol. 19:173–179 [Google Scholar]
- 6. Bradford M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 7. Chand-Goyal T., Spotts R. A. 1996. Enumeration of bacterial and yeast colonists of apple fruits and identification of epiphytic yeasts on pear fruits in the Pacific Northwest United States. Microbiol. Res. 151:427–432 [DOI] [PubMed] [Google Scholar]
- 8. Chen Y., Know S. W., Kim S. C., Zhao Y. 2005. Integrated approach for manual evaluation of peptides identified by searching protein sequence databases with tandem mass spectra. J. Proteome Res. 4:998–1005 [DOI] [PubMed] [Google Scholar]
- 9. Church F. C., Swaisgood H. E., Porter D. H., Catignani G. L. 1983. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 66:1219–1227 [Google Scholar]
- 10. Coda R., et al. 2008. Long-term fungi inhibitory activity of water-soluble extract from Phaseolus vulgaris cv. Pinto and sourdough lactic acid bacteria during bread storage. Appl. Environ. Microbiol. 74:7391–7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coda R., et al. 2010. Spelt and emmer flours: characterization of the lactic acid bacteria microbiota and selection of mixed starters for bread making. J. Appl. Microbiol. 108:925–935 [DOI] [PubMed] [Google Scholar]
- 12. Corsetti A., Gobbetti M., Rossi J., Damiani P. 1998. Antimould activity of sourdough lactic acid bacteria: identification of a mixture of organic acids produced by Lactobacillus sanfrancisco CB1. Appl. Microbiol. Biotechnol. 50:253–256 [DOI] [PubMed] [Google Scholar]
- 13. Crowley P., Schober T., Clarke C., Arendt E. 2002. The effect of storage time on textural and crumb grain characteristics of sourdough wheat bread. Eur. Food Res. Technol. 214:489–496 [Google Scholar]
- 14. Daniel H. M., Moons M.-C., Huret S., Vrancken G., De Vuyst L. 2010. Wickerhamomyces anomalus in the sourdough microbial ecosystem. Antonie Van Leeuwenhoek. doi: 10.1007/s10482-010-9517-2 [DOI] [PubMed] [Google Scholar]
- 15. Dantigny P., Guilmart A., Radoi F., Bensoussan M., Zwietering M. 2005. Modelling the effect of ethanol on growth rate of food spoilage moulds. Int. J. Food Microbiol. 98:261–269 [DOI] [PubMed] [Google Scholar]
- 16. Druvefors U. A., Passoth V., Schnürer J. 2005. Nutrient effects on biocontrol of Penicillium roqueforti by Pichia anomala J121 during airtight storage of wheat. Appl. Environ. Microbiol. 71:1865–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Egorov T. A., Odintsova T. I., Pukhalsky V. A., Grishin E. V. 2005. Diversity of wheat anti-microbial peptides. Peptides 26:2064–2073 [DOI] [PubMed] [Google Scholar]
- 18. European Union 1995. European Parliament and Council directive no. 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners, p. 53 European Union, Brussels, Belgium: http://europa.eu.int/eur-lex/en/consleg/pdf/1995/en_1995L0002_do_001.pdf [Google Scholar]
- 19. Gobbetti M., Corsetti A., Rossi J. 1994. The sourdough microflora. Interactions between lactic acid bacteria and yeasts: metabolism of carbohydrates. Appl. Microbiol. Biotechnol. 41:456–460 [DOI] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21. Haglund Å., Johansson L., Dahlstedt L. 1998. Sensory evaluation of wholemeal bread from ecologically and conventionally grown wheat. J. Cereal Sci. 27:199–207 [Google Scholar]
- 22. Reference deleted.
- 23. Lavermicocca P., et al. 2000. Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl. Environ. Microbiol. 66:4084–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magnusson J., Schnürer J. 2001. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 67:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magnusson J., Ström K., Roos S., Sjögren J., Schnürer J. 2003. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol. Lett. 219:129–135 [DOI] [PubMed] [Google Scholar]
- 26. Reference deleted.
- 27. Niku-Paavola M., Laitila L. A., Mattila-Sandholm T., Hikara A. 1999. New types of antimicrobial compounds produced by Lactobacillus plantarum. J. Appl. Microbiol. 86:29–35 [DOI] [PubMed] [Google Scholar]
- 28. Okkers D. J., Dicks L. M. T., Silvester M., Joubert J. J., Odendaal H. J. 1999. Characterization of pentocin TV35b, a bacteriocin-like peptide isolated from Lactobacillus pentosus with a fungistatic effect on Candida albicans. J. Appl. Microbiol. 87:726–734 [DOI] [PubMed] [Google Scholar]
- 29. Osborne T. B. 1907. The proteins of the wheat kernel. Carnegie Institute of Washington publication 84. Judd and Detweiler, Washington, DC [Google Scholar]
- 30. Passoth V., Fredlund E., Druvefors U. Ä., Schnürer J. 2006. Biotechnology, physiology and genetics of the yeast Pichia anomala. FEMS Yeast Res. 6:3–13 [DOI] [PubMed] [Google Scholar]
- 31. Pattison T. L., Lindsay D., von Holy A. 2004. Natural antimicrobial as potential replacements for calcium propionate in bread. S. Afr. J. Sci. 100:339–342 [Google Scholar]
- 32. Petersson S., Schnurer J. 1995. Biocontrol of mold growth in high-moisture wheat stored under airtight conditions by Pichia anomala, Pichia guilliermondii, and Saccharomyces cerevisiae. Appl. Environ. Microbiol. 61:1027–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reference deleted.
- 34. Petersson S., Jonsson N., Schnürer J. 1999. Pichia anomala as a biocontrol agent during storage of high-moisture feed grain under airtight condition. Postharvest Biol. Technol. 15:175–184 [Google Scholar]
- 35. Piano S., Neyrotti V., Migheli Q., Gullino M. L. 1997. Biocontrol capability of Metschnikowia pulcherrima against Botrytis postharvest rot of apple. Postharvest Biol. Technol. 11:131–140 [Google Scholar]
- 36. Quiroga E. N., Sampietro A. R., Vattuone M. A. 2001. Screening antifungal activities of selected medicinal plants. J. Ethnopharmacol. 74:89–96 [DOI] [PubMed] [Google Scholar]
- 37. Rizzello C. G., Cassone A., Coda R., Gobbetti M. 2011. Antifungal activity of sourdough fermented wheat germ used as an ingredient for bread making. Food Chem. 127:952–959 [DOI] [PubMed] [Google Scholar]
- 38. Rizzello C. G., Nionelli L., Coda R., De Angelis M., Gobbetti M. 2010. Effect of sourdough fermentation on stabilization, and chemical and nutritional characteristics of wheat germ. Food Chem. 119:1079–1089 [Google Scholar]
- 39. Rizzello C. G., Nionelli L., Coda R., Di Cagno R., Gobbetti M. 2010. Use of sourdough fermented wheat germ for enhancing the nutritional, texture and sensory characteristics of the white bread. Eur. Food Res. Technol. 230:645–654 [Google Scholar]
- 40. Rizzello C. G., et al. 2009. Long-term fungal inhibitory activity of water-soluble extract from Amaranthus spp. seeds during storage of gluten-free and wheat flour breads. Int. J. Food Microbiol. 131:189–196 [DOI] [PubMed] [Google Scholar]
- 41. Selitrennikoff C. P. 2001. Antifungal proteins. Appl. Environ. Microbiol. 67:2883–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sjögren J., Magnusson J., Broberg A., Schnürer J., Kenne L. 2003. Antifungal 3-hydroxy fatty acids from Lactobacillus plantarum MiLAB 14. Appl. Environ. Microbiol. 69:7554–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ström K., Sjögren J., Broberg A., Schnürer J. 2002. Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(l-Phe-l-Pro) and cyclo(l-Phe-l-trans-4-OH-l-Pro) and β-phenyllactic acid. Appl. Environ. Microbiol. 68:4322–4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swiegers J. H., Bartowsky E. J., Henschke P. A., Pretorius I. S. 2005. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 11:139–173 [Google Scholar]
- 45. Walker G. M., Mcleod A. H., Hodgson V. J. 1995. Interactions between killer yeasts and pathogenic fungi. FEMS Microbiol. Lett. 127:213–222 [DOI] [PubMed] [Google Scholar]
- 46. Wang H., Ng T. B. 2001. Novel antifungal peptides from Ceylon spinach seeds. Biochem. Biophys. Res. Commun. 288:765–770 [DOI] [PubMed] [Google Scholar]
- 47. Wang Z., Wang G. 2004. APD: the Antimicrobial Peptide Database. Nucleic Acids Res. 32:590–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weiss W., Vogelmeier C., Gorg A. 1993. Electrophoretic characterization of wheat grain allergens from different cultivars involved in bakers' asthma. Electrophoresis 14:805–816 [DOI] [PubMed] [Google Scholar]