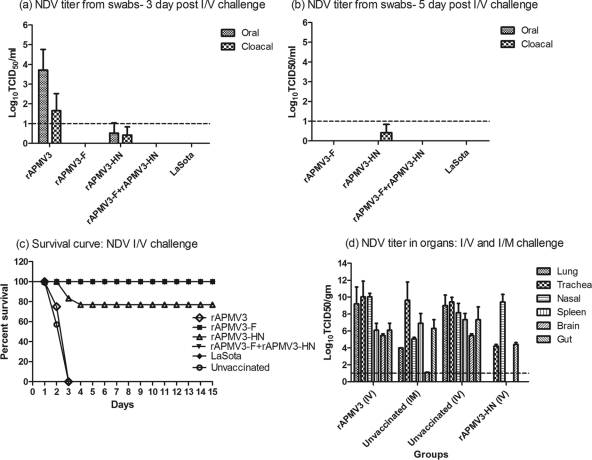
Fig. 10.
Protective efficacy of rAPMV3s immunization after intravenous challenge. Shown is the NDV challenge virus shedding titer as determined by TCID50 assay in oral and cloacal swabs on 3, 5 and 7 days after intravenous (I/V) challenge of chicken groups immunized with rAPMV3s. No virus was detected in the rAPMV3-F-, rAPMV3-F-plus-rAPMV3-HN-, and LaSota-vaccinated groups 3 days after intravenous challenge (a). Furthermore, no virus shedding was observed 5 days after intravenous challenge, except for cloacal shedding in the rAPMV3-HN group (b). No virus shedding was observed 7 days after intravenous challenge. The percent survival of rAPMV3-immunized chickens following NDV challenge by intravenous routes is shown in panel c. All of the unvaccinated control and rAPMV3 empty vector-infected chickens and 4 out of 5 rAPMV3-HN-infected chickens died after intravenous challenge by day 3. All of the chickens in the rAPMV3-F, rAPMV3-F-plus-rAPMV3-HN, and LaSota groups survived after intravenous challenge. Statistical differences between the groups were determined by the log-rank test with P < 0.0001. Panel d shows replication of NDV challenge virus after intramuscular (I/M) and intravenous challenge in different organs of chickens 21 days after immunization with rAPMV3s. Various organs from each chicken that died postchallenge were collected, and virus was analyzed in DF1 cells. The NDV titers in different organs of each chicken are expressed in log10 TCID50 per g of tissue (d).