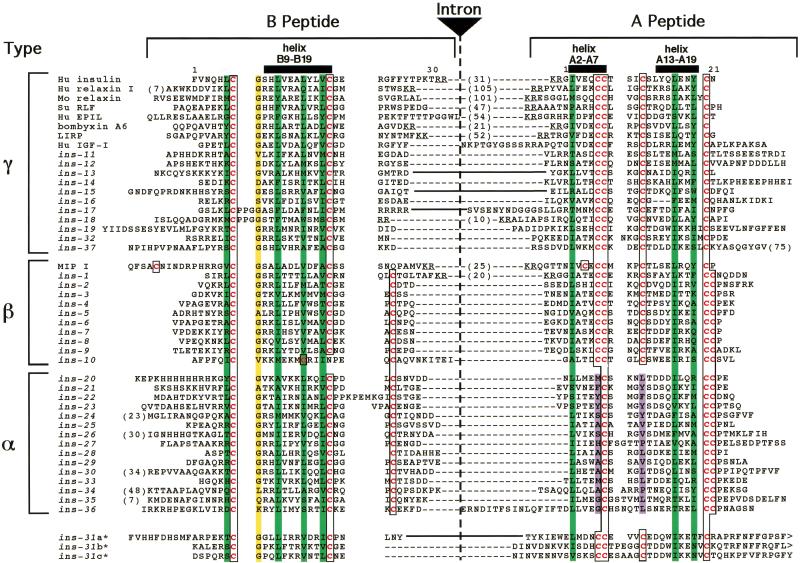
Figure 2.
Sequence diversity of ins genes. Sequence alignment of B and A domains. Cysteine residues are in red type and boxed. Hydrophobic residues important for helix formation are overlaid in green. The conserved glycine at B8 is overlaid in yellow. The nonpolar residues that replace the A16/A11 disulfide bond are overlaid in purple. An intron is inserted at the end of the B domain except where indicated by solid lines between the domains. The sizes (in residues) of cleaved or potentially cleavable C domains are indicated by numbers in parentheses between the B and A domain sequences. Actual or predicted dibasic residue cleavage sites for prohormone convertase are underlined. The ins genes defined previously are ins-2 through ins-7, ins-11, and ins-21 through ins-23 (Duret et al. 1998), ins-1 (Gregoire et al. 1998), and ins-18 (Kawano et al. 2000).