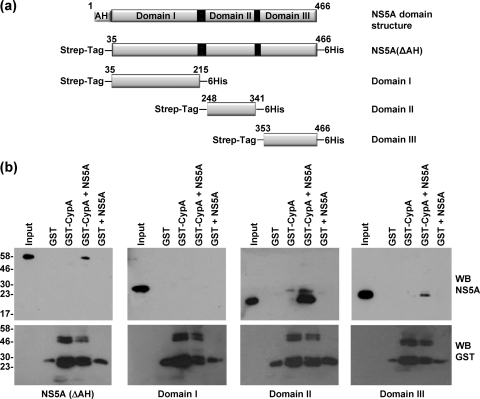
Fig. 1.
GST-CypA interacts with domains II and III, but not domain I, of NS5A. (a) Schematic diagram of the functional domains of NS5A and the design of the constructs used in the article (JFH-1 genotype 2a NS5A protein numbering). The NS5A constructs all lacked the N-terminal amphipathic helix (AH) and contained an N-terminal Strep tag and a C-terminal hexahistidine tag (6). The full-length construct is referred to as NS5A(ΔAH). (b) Purified GST, or GST-CypA (40 μg), was immobilized on 20 μl glutathione-agarose beads and incubated with 5 μg purified NS5A(ΔAH) or individual domains overnight at 4°C in binding buffer (40 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 10 mm dithiothreitol [DTT], 50 μg/ml bovine serum albumin [BSA], 10 μg/ml yeast tRNA [Ambion]) (6). Beads were washed three times, eluted in SDS-PAGE sample buffer, and analyzed by Western blotting (WB) with a sheep polyclonal antibody to NS5A (16) or a mouse anti-GST antibody (Serotec).