Abstract
Farnesoid X receptor (FXR), the primary bile acid-sensing nuclear receptor, also is known for its anticancer properties. It is known that FXR deficiency in mice results in spontaneous hepatocellular carcinoma (HCC), but the mechanisms are not completely understood. We report that sustained activation of the Wnt/β-catenin pathway is associated with spontaneous HCC in FXR-knockout (KO) mice. HCC development was studied in FXR-KO mice at 3, 8, and 14 months of age. No tumors were observed at either 3 or 8 months, but the presence of HCC was observed in 100% of the FXR-KO mice at the age of 14 months. Further analysis revealed no change in β-catenin activation in the livers of 3-month-old FXR-KO mice, but a moderate increase was observed in 8-month-old FXR-KO mice. β-Catenin activation further increased significantly in 14-month-old tumor-bearing mice. Further analysis revealed that two independent mechanisms might be involved in β-catenin activation in the livers of FXR-KO mice. Activation of canonical Wnt signaling was evident as indicated by increased Wnt4 and dishevelled expression along with glycogen synthase kinase-3β inactivation. We also observed decreased expression of E-cadherin, a known regulator of β-catenin, in FXR-KO mice. The decrease in E-cadherin expression was accompanied by increased expression of its transcriptional repressor, Snail. Consistent with the increased HCC in FXR-KO mice, we observed a significant decrease in FXR expression and activity in human HCC samples. Taken together, these data indicate that a temporal increase in the activation of Wnt/β-catenin is observed during spontaneous HCC development in FXR-KO mice and is potentially critical for tumor development.
Introduction
Farnesoid X receptor (FXR) is the main bile acid-sensing receptor in the body and expressed at high levels in the liver and gut (Forman et al., 1995; Sinal et al., 2000; Wang et al., 2008). The role of FXR has been recognized in a variety of physiological and pathological processes, including the regulation of bile acid homeostasis (Guo et al., 2003; Lambert et al., 2003; Eloranta and Kullak-Ublick, 2008; Gadaleta et al., 2010), lipid metabolism, liver regeneration, inflammation, and cancer (Huang et al., 2006; Modica et al., 2008; Wang et al., 2008). It is known that the loss of FXR as observed in whole-body FXR knockout (FXR-KO) mice results in increased carcinogenesis of the colon and the liver (Kim et al., 2007b; Yang et al., 2007; Maran et al., 2009). FXR-KO mice develop spontaneous hepatocellular carcinoma (HCC) at the age of 12 to 14 months, but the mechanisms remain unknown (Kim et al., 2007b; Yang et al., 2007). It is known that FXR-KO mice have 4-fold higher total bile acids, and a decrease in bile acids using cholestyramine has been shown to decrease HCC incidence in FXR-KO mice (Yang et al., 2007). However, the exact role of FXR or a subsequent increase in bile acids in the pathogenesis of HCC is not known.
The Wnt/β-catenin pathway plays a central role in liver biology and is involved in embryonic and postnatal liver development, liver regeneration, hepatic progenitor cell biology, and pathogenesis of liver cancer (Thompson and Monga, 2007). Mutations in CTNNB1, the gene that encodes the β-catenin protein, the downstream effector of the Wnt/β-catenin pathway, are observed in a large portion of cases of HCC. Furthermore, increased β-catenin activation is observed in a majority of cases of HCC (Carruba et al., 1999; Huang et al., 1999; Devereux et al., 2001; Fujie et al., 2001; Calvisi et al., 2004a). A substantial increase in the activation of β-catenin also has been observed in hepatoblastomas, the primary hepatic malignancy in children (Taniguchi et al., 2002; Monga et al., 2003). Furthermore, increased expression of Wnt proteins, the extracellular ligands of the Wnt/β-catenin pathway, and decreased expression of dickkopf-1 and soluble frizzled-related protein, the inhibitors of Wnt signaling, have been observed in various cancers (Kolligs et al., 2002).
We hypothesized that spontaneous hepatocarcinogenesis in FXR-KO mice is associated with β-catenin activation. We investigated the activation of the Wnt/β-catenin pathway during tumorigenesis in FXR-KO mice over a time course of 3 to 14 months. Our studies demonstrate sustained activation of β-catenin and reveal the mechanisms behind increased β-catenin activation in the livers of FXR-KO mice.
Materials and Methods
Antibodies.
The primary antibodies used in these studies were as follows: mouse anti-β-catenin (BD Biosciences, San Jose, CA), mouse anti-activated β-catenin (Millipore, Billerica, MA), rabbit anti-glycogen synthase kinase 3β (GSK-3β), rabbit anti-Ser9-phospho-GSK-3β, rabbit anti-Ser45-Thr41-phospho-β-catenin, rabbit anti-E-cadherin, rabbit anti-Snail, and rabbit anti-dishevelled (Dvl) (Cell Signaling Technology, Danvers, MA), Wnt4 (Santa Cruz Biotechnology, Santa Cruz, CA). All of the Western blotting secondary antibodies were purchased from Cell Signaling Technology, and biotinylated secondary antibodies for immunohistochemistry were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Animals and Tissue Harvesting.
Three-month-old (n = 5), 8-month-old (n = 5), 12- to 14-month old FXR-KO (n = 17), and wild-type (WT) (C57BL/6, n = 10) mice were used in these studies. FXR-KO mice used in these studies are backcrossed into the C57BL/6 genetic background for 10 generations and have been described in detail previously (Maran et al., 2009). All of the animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities at the University of Kansas Medical Center under a standard 12-h light/dark cycle with access to chow and water ad libitum. The Institutional Animal Care and Use Committee approved all of the studies. Mice were killed by cervical dislocation under isoflurane anesthesia, and livers were collected. Pieces of liver were fixed in 10% neutral buffered formalin for 48 h and further processed to obtain paraffin blocks, and 4-μm-thick sections were obtained. A piece of liver was frozen in optimum cutting temperature and used to obtain fresh frozen sections. A part of the liver tissue was used to prepare fresh nuclear and cytoplasmic protein extracts using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Waltham, MA). The remaining liver tissue was frozen in liquid N2 and stored at −80°C until used to prepare radioimmunoprecipitation assay (RIPA) extracts.
Protein Isolation and Western Blotting.
Total protein was isolated from livers of WT and FXR-KO mice using RIPA buffer [1% SDS, 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% NP-40, 1% Triton X-100, and 0.25% sodium deoxycholate]. Protease and phosphatase inhibitors (Halt Protease and Phosphatase Inhibitor Cocktail with EDTA; Thermo Fisher Scientific) at a concentration of 1:100 were freshly added to the RIPA buffer before use. Cell lysates were prepared using glass homogenizers. Protein concentrations of all of the lysates were determined using the bicinchoninic acid protein assay reagents (Thermo Fisher Scientific). Total cell lysates made in RIPA buffer (50 μg) were separated by electrophoresis on 4 to 12% NuPage Bis-Tris gels with MOPS buffer (Invitrogen, Carlsbad, CA), then transferred to Immobilon-P membranes (Millipore) in NuPAGE transfer buffer containing 20% methanol. Membranes were stained with Ponceau S to verify loading and transfer efficiency. Membranes were probed with primary and secondary antibodies in Tris-buffered saline with Tween 20 containing either 5% nonfat milk or 5% bovine serum albumin depending on the antibody used. Signal was visualized by incubating the blots in SuperSignal West Pico Chemiluminescence Substrate (Thermo Fisher Scientific) and exposing to X-ray film (MidSci, St. Louis, MO).
Immunohistochemistry and Immunofluorescence.
Paraffin-embedded liver sections (4 μm in thickness) were used for immunohistochemical staining. Antigen retrieval was achieved by the citrate buffer method. Slides were placed in boiling citrate buffer solution for 5 min followed by 10 min at subboiling temperature. The tissue sections were blocked in either 5% normal goat serum for 30 min or Ultra V Block solution (Thermo Fisher Scientific) for 5 min followed by incubation with the pertinent primary antibody overnight at 4°C. The primary antibody then was linked to the biotinylated secondary antibody followed by the routine avidin–biotin complex method (ABC Vectastain kit; Vector Laboratories, Burlingame, CA). Diaminobenzidine was used as the chromogen, which resulted in a brown reaction product. Immunofluorescence staining of β-catenin was performed using fresh frozen sections as described previously (Monga et al., 2006).
Real-Time Polymerase Chain Reaction.
To quantify Wnt and frizzled (Fzl) mRNA levels, TaqMan-based real-time polymerase chain reaction (PCR) arrays (Applied Biosystems, Foster City, CA) were used. Total RNA was isolated from the livers of 8- and 14-month-old WT and FXR-KO mice using the TRIzol method according to the manufacturer's protocol (Sigma-Aldrich, St. Louis, MO) and converted to cDNA as described previously (Apte et al., 2009). Real-time PCR was performed on a StepOnePlus real-time PCR machine (Applied Biosystems). Wnt and Fzd gene expression was normalized to 18S ribosomal RNA and glyceraldehyde-3-phosphate dehydrogenase gene expression in the same samples.
FXR, small heterodimer partner (SHP), and bile salt export pump (BSEP) mRNA levels were quantified in normal and HCC human cDNA purchased from Origene (Rockville, MD). These cDNA samples have been obtained from a total of 34 HCC and 8 normal livers verified by pathologists before isolation of RNA and conversion to cDNA. The HCC samples included seven samples of stage I HCC, eight samples of stage II and IIIA HCC each, and three samples of stage IV HCC (Supplemental Table 1). To study FXR, SHP, and BSEP gene expression, SYBR Green-based (Applied Biosystems) real-time PCR was conducted using the Applied Biosystems Prism 7300 real-time PCR instrument as described previously (Thomas et al., 2010). FXR, BSEP, and SHP gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase gene expression. The primers used in this study are described in Table 1. The specificity of these primers was verified both by agarose gel analysis and by reviewing the melting and amplification curves during real-time PCR.
TABLE 1.
Primers used for real-time PCR analysis
| Gene Symbol | 5′ Primer Sequence | 3′ Primer Sequence |
|---|---|---|
| hGAPDH | 5′-GGTGGTCTCCTCTGACTTCAA-3′ | 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ |
| hFXR | 5′-TGCATTGAAGTTGCTCTCAGGT-3′ | 5′-CGCCTGACTGAATTACGGACA-3′ |
| hBSEP | 5′-AGTTGCTCATCGCTTGTCTACG-3′ | 5′-GCTTGATTTCCCTGGCTTTG-3′ |
| hSHP | 5′-AGCTGGAAGTGAGAGCAGATCC-3′ | 5′-AGAAGTGCGTAGAGAATGGCG-3′ |
Statistical Analysis.
Data presented in the form of bar graphs show mean ± S.D. To determine statistically significant differences between groups, a paired Student's t test was used. Difference between groups was considered statistically significant at P < 0.05. The different degrees of significance were indicated as follows in the bar graphs: *, P < 0.5; **, P < 0.01; ***, P < 0.001.
Results
Extensive Hepatic Tumor Development in FXR-KO Mice.
We observed extensive hepatic tumors in 100% of 12- to 14-month-old FXR-KO mice, whereas none of the age-matched WT mice had any tumors. Hematoxylin and eosin staining of paraffin slides indicated normal histology with well organized hepatic plates radiating from the central vein to the portal areas in the WT mice (Supplemental Fig. 1, top panel). In FXR-KO mice, the hepatic architecture was distorted severely due to tumor formation. Livers of FXR-KO mice had HCC as indicated by the presence of disorganized clear cells, highly eosinophilic cells, and inflammatory cells in some tumors. Proliferating cell nuclear antigen immunohistochemistry indicated less than 0.1% cells in cell cycle in the WT livers (Supplemental Fig. 1, bottom panel), whereas significant proliferation was observed in the livers of FXR-KO mice. The rate of cell proliferation differed in the tumors of FXR-KO mice from moderate (Supplemental Fig. 1, bottom panel, middle photograph) to very high (Supplemental Fig. 1, bottom panel, right photograph).
Increased β-Catenin Activation in Livers of FXR-KO Mice.
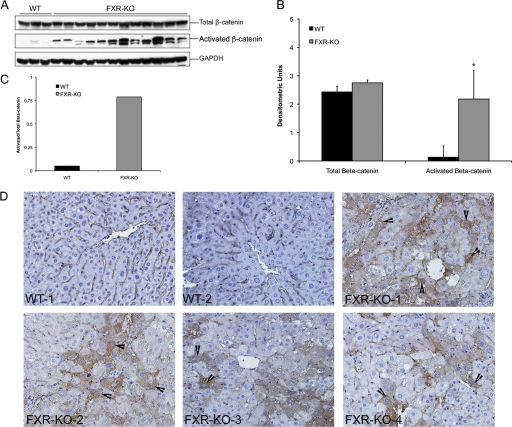
To determine whether β-catenin activation was observed in the HCC in livers of 12- to 14-month-old FXR-KO mice, we performed a Western blot for total and activated β-catenin (dephosphorylated form known to translocate to the nucleus). The Western blot analysis indicates a moderate increase in total β-catenin protein but a significant increase in activated β-catenin in the livers of FXR-KO mice compared with those of the livers of WT mice (Fig. 1, A and B). The ratio of activated to total β-catenin expression indicated 15-fold higher β-catenin activation in the FXR-KO mice (Fig. 1C). Immunohistochemical staining of total β-catenin revealed mainly membranous staining on hepatocytes in WT mice with no apparent staining for activated β-catenin (Fig. 1D). In contrast, foci of β-catenin-positive cells with extensive cytoplasmic stabilization and nuclear translocation of β-catenin were observed in the livers of FXR-KO mice.
Fig. 1.
Increased activation of β-catenin in the livers of FXR-KO mice. A, Western blot analysis for total and activated (dephosphorylated) β-catenin using total liver extracts from WT and FXR-KO mice. B, densitometric analysis of total and activated β-catenin Western blots from liver homogenates from WT and FXR-KO mice in A. Data are expressed as mean ± S.D. Statistical significance: *, P < 0.05. C, bar graph showing the ratio of activated to total β-catenin in the livers of WT and FXR-KO mice. D, representative photomicrographs of total β-catenin immunohistochemistry in the livers of WT and FXR-KO mice. Representative photographs of the livers of two WT and four independent FXR-KO mice are shown. Arrowheads point to cytoplasmic and nuclear stabilization of β-catenin. Magnification, 400×.
Increased β-Catenin Nuclear Localization and Target Gene Expression in Livers of FXR-KO Mice.
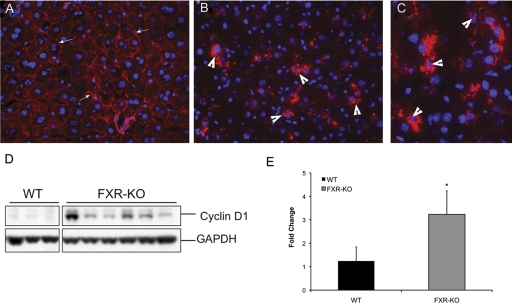
We further investigated nuclear localization of β-catenin using the immunofluorescence staining method. Immunofluorescence analysis indicated membranous β-catenin staining in WT mice, whereas extensive cytoplasmic stabilization and nuclear localization of β-catenin were evident in the livers of FXR-KO mice (Fig. 2, A–C). To investigate the functional significance of β-catenin activation in the livers of FXR-KO mice, we determined the levels of the β-catenin target gene cyclin D1. Real-time PCR and Western blot analysis (Fig. 2, D and E) showed increased mRNA and protein levels of cyclin D1 in the livers of FXR-KO mice.
Fig. 2.
β-Catenin activation results in cyclin D1 up-regulation and is regulated by canonical signaling in FXR-KO mice. A–C, immunofluorescence staining of total β-catenin in WT (A) and FXR-KO mice (B, magnification 400×; C, magnification 600×). Arrowheads point to cytoplasmic and nuclear stabilization of β-catenin. D, Western blot analysis of cyclin D1 using total liver homogenates of WT and FXR-KO mice. E, real-time PCR analysis of cyclin D1 in livers of WT and FXR-KO mice. Data are expressed as mean ± S.D. Statistical significance: *, P < 0.05.
Activation of β-Catenin Is Secondary to GSK-3β Inactivation.
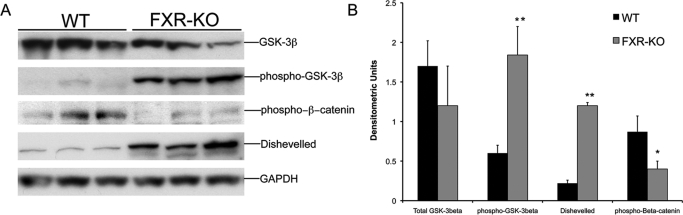
To identify the mechanism behind β-catenin activation in the FXR-KO mice, we estimated the levels of GSK-3β, the main negative regulator of β-catenin in the cells. Western blot analysis (Fig. 3) indicated a moderate decrease in total GSK-3β protein, which was not statistically significant. However, a 3-fold increase in Ser9-phosphorylated (inactive) GSK-3β was observed in the livers of FXR-KO mice, indicating marked GSK-3β inactivation in FXR-KO mice. In the canonical Wnt signaling pathway, an upstream multimodule protein called Dvl plays a critical role in the regulation of GSK-3β activity. A Western blot analysis of Dvl indicated that the livers of FXR-KO mice had a 4-fold increase in total Dvl protein compared with that of livers of WT mice. Furthermore, a decrease in Ser45-Thr41-phosphorylated (inactive) β-catenin was observed in the livers of FXR-KO mice, which is consistent with increased activation of β-catenin.
Fig. 3.
Increased GSK-3β inactivation in FXR-KO mice. A, Western blot analysis of total and Ser9-phosphorylated GSK-3β, Ser45-Thr41-phosphorylated β-catenin, and Dvl performed using liver homogenates from WT and FXR-KO mice. B, densitometric analysis of blots in A. Data are expressed as mean ± S.D. Statistical significance: *, P < 0.05; **, P < 0.01.
Decreased E-Cadherin Expression in FXR-KO Mice.
β-Catenin activation is known to be associated with a decrease in E-cadherin expression during carcinogenesis. To study whether the loss of E-cadherin may be involved in the pathogenesis of HCC in the livers of FXR-KO mice, we estimated the levels of E-cadherin in the livers of FXR-KO and WT mice. Western blot analysis and immunohistochemical staining (Fig. 4, A and C) revealed a substantial decrease in E-cadherin in the livers of FXR-KO mice compared with that in the livers of WT mice. It has been shown that the transcriptional repressor Snail can negatively regulate E-cadherin during tumorigenesis, a process referred to as epithelial-to-mesenchymal transition. To study whether the decreased E-cadherin levels observed in the livers of FXR-KO mice are associated with an increase in Snail, we estimated the levels of Snail protein by Western blot analysis. The livers of FXR-KO mice had a 2-fold up-regulation of Snail compared with that of the livers of WT mice, which is consistent with a decrease in E-cadherin expression (Fig. 4, A and B).
Fig. 4.
Loss of E-cadherin as a mechanism of β-catenin activation. A, Western blots of E-cadherin and Snail conducted using liver homogenates from WT and FXR-KO mice. B, densitometric analysis of E-cadherin and Snail Western blots. Western blots from three separate mice were scanned and used for the densitometric analysis. Data are expressed as mean ± S.D. Statistical significance: ***, P < 0.001. C, representative photographs of E-cadherin immunohistochemistry on paraffin sections of livers from WT and FXR-KO mice. Arrowheads indicate E-cadherin staining on cell membranes.
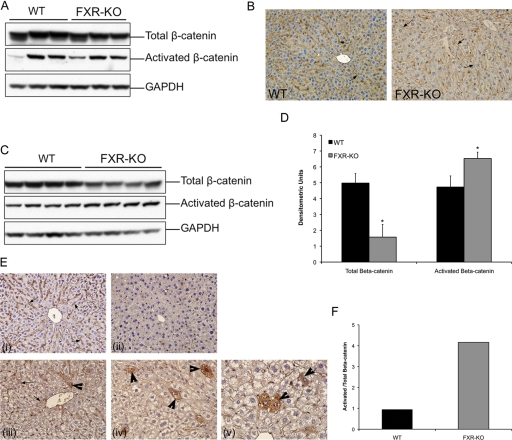
Temporal Activation of β-Catenin in the Livers of FXR-KO Mice.
Our data indicate that β-catenin activation is observed in HCC-bearing livers of FXR-KO mice at 12 to 14 months of age. To determine whether β-catenin activation occurs during the tumorigenesis process, we investigated the livers of 3- and 8-month-old WT and FXR-KO mice. Hematoxylin and eosin staining revealed no change in the livers of WT mice, but a moderate dysplasia was observed in the livers of FXR-KO mice (data not shown). β-Catenin staining and Western blot analysis showed no change in total or activated β-catenin at 3 months of age in the FXR-KO mice compared with that in the WT mice (Fig. 5, A and B). However, a moderate decrease in total β-catenin and a moderate increase in activated β-catenin were observed in the livers of 8-month-old FXR-KO mice compared with those of age-matched WT mice (Fig. 5, C and D). The ratio of activated to total β-catenin indicated 4-fold higher β-catenin activation in the livers of FXR-KO mice at 8 months of age (Fig. 5F). Immunohistochemical staining revealed numerous foci of hepatocytes with cytoplasmic stabilization and nuclear translocation of β-catenin in the livers of 8-month-old FXR-KO mice (Fig. 5E).
Fig. 5.
Temporal increase in β-catenin activation in the livers of FXR-KO mice. A, Western blot analysis of total and activated β-catenin in the livers of 3-month-old WT and FXR-KO mice. B, representative photomicrographs of β-catenin immunohistochemistry in the livers of 3-month-old WT and FXR-KO mice. Arrows indicate membranous β-catenin staining. C, Western blot analysis of total and activated β-catenin in the livers of 8-month-old WT and FXR-KO mice. D, densitometry of blots in C. Data are expressed as mean ± S.D. Statistical significance: *, P < 0.05. E, representative photomicrographs of immunohistochemical staining for total β-catenin (i) and activated β-catenin (ii) in WT mice and total β-catenin (iii) and activated β-catenin (iv) in FXR-KO mice. A high-magnification (600×) photomicrograph of activated β-catenin immunohistochemistry in the livers of FXR-KO mice at 8 months of age (v). F, bar graph showing the ratio of activated to total β-catenin in the livers of 8-month-old WT and FXR-KO mice.
Increased Wnt4 and Fzl Expression in the Livers of FXR-KO Mice.
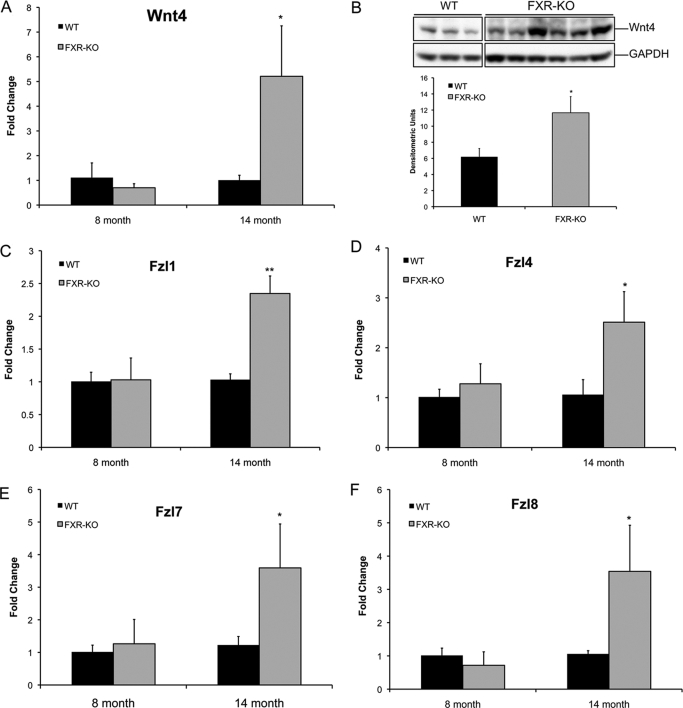
GSK-3β inactivation and Dvl up-regulation in the FXR-KO mice suggest that increased β-catenin activation in the livers of FXR-KO mice could be due to the activation of the canonical Wnt pathway. The canonical Wnt signaling is initiated by secreted Wnt proteins, which bind to the Fzl receptors. A total of 19 Wnt and 10 Fzl genes are functional in mammals, of which 11 Wnt and 8 Fzl genes are expressed in the liver (Zeng et al., 2007). We determined mRNA expression of all 19 Wnt and 10 Fzl genes using TaqMan-based real-time PCR arrays in the livers of 8- and 14-month-old WT and FXR-KO mice (Fig. 6). Our analysis indicates that Wnt4 was the only Wnt gene that was expressed 5-fold higher in the 14-month-old FXR-KO mice (Fig. 6A). Western blot analysis indicated increased Wnt protein expression in the FXR-KO mice at 14 months of age (Fig. 6B). Furthermore, we observed induced expression of Fzl1, Fzl4, Fzl7, and Fzl8 mRNA (all 2.5-fold higher) in the livers of 14-month-old FXR-KO mice (Fig. 6, C–F). These data indicate that Wnt4 may play a crucial role in the stimulation of canonical Wnt signaling in FXR-KO mice.
Fig. 6.
Increased Wnt and Fzl expression in FXR-KO mice. A, real-time PCR analysis of Wnt4 in the livers of 8- and 14-month-old WT and FXR-KO mice. B, Western blot analysis and densitometric analysis of the Western blots for Wnt4 in the livers of 14-month-old WT and FXR-KO mice. C–F, real-time PCR analysis of Fzl1 (C), Fzl4 (D), Fzl7 (E), and Fzl8 (F) in 8- and 14-month-old WT and FXR-KO mice. Data are expressed as mean ± S.D. Statistical significance: *, P < 0.05; **, P < 0.01.
Decreased FXR Expression and Activity in Human HCC.
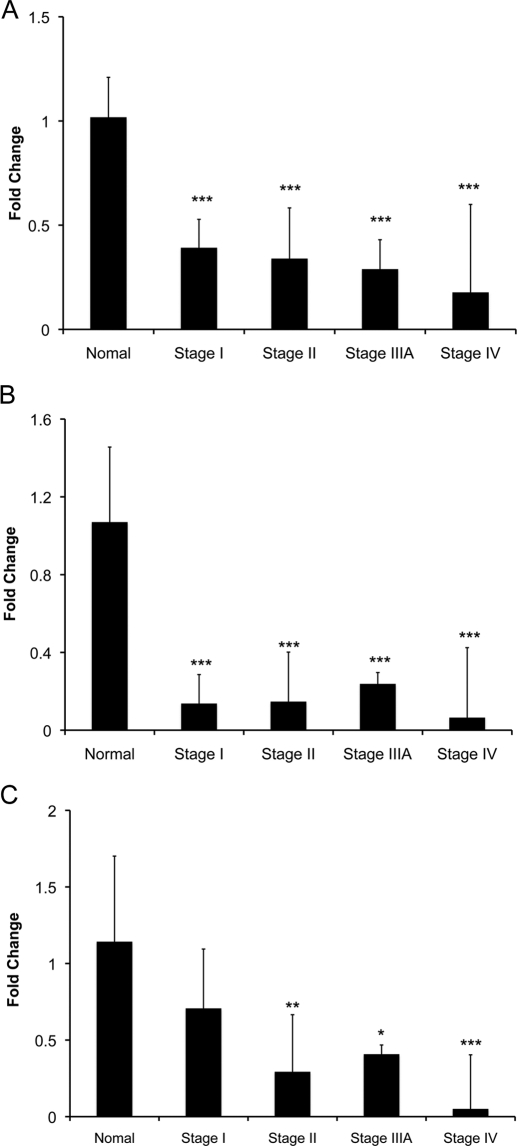
To investigate whether the loss of FXR is relevant to HCC pathogenesis in humans, we investigated FXR gene expression and function in human HCC samples and normal livers by real-time PCR. The data indicated that FXR expression was decreased to 40% of normal as early as in stage I of HCC (Fig. 7A) and decreased further in stage II, IIIA, and IV (20% of normal). To assess the change in FXR function, we estimated the gene expression of SHP and BSEP, two well characterized target genes of FXR (Fig. 7B). The data revealed that SHP expression decreased to 20% of normal in stages I to IV of HCC. No difference in BSEP expression was observed in stage I of HCC, but BSEP expression decreased to 10% of normal in stages II, IIIA, and IV of HCC.
Fig. 7.
Decreased FXR expression and activity in human HCC. A total of 40 HCC and 8 normal liver samples were studied. FXR (A), SHP (B), and BSEP (C) mRNA expression detected by real-time PCR using normal liver samples and liver samples of various stages of HCC. Data are expressed as mean ± S.D. Statistical significance: *, P < 0.05; **, P < 0.01; ***, P < 0.001. All of the comparisons are between the normal livers and the respective stage of HCC.
Discussion
HCC is the most common hepatic malignancy, with extremely grim prognosis and limited treatment options (El-Serag and Mason, 1999; Befeler and Di Bisceglie, 2002; Di Bisceglie and Befeler, 2007). Whereas the mechanisms of HCC pathogenesis are not completely clear, it has been observed that signaling cross-talk between multiple signal transduction pathways is involved in the progression of HCC (Aravalli et al., 2008; Llovet and Bruix, 2008). Our studies indicate that signaling cross-talk among bile acids, FXR, and the Wnt/β-catenin pathway may be involved in the pathogenesis of HCC.
Previous studies have demonstrated that the loss of FXR results in increased tumorigenesis in the colon and liver (Kim et al., 2007b; Yang et al., 2007; Maran et al., 2009). Whereas two independent groups have observed spontaneous HCC in FXR-KO mice, the mechanisms are not completely clear. We observed spontaneous HCC development in all of the FXR-KO mice studied at the ages of 12 to 14 months, thus corroborating previous findings. Because Wnt/β-catenin signaling has been implicated in the pathogenesis of HCC, we investigated whether the activation of β-catenin occurs in the livers of FXR-KO mice during HCC formation. Our data indicate a significant increase in β-catenin activation in the tumors of FXR-KO mice. We also observed a temporal increase in β-catenin activation in the livers of FXR-KO mice during the tumorigenesis process. Kim et al. (2007b) have previously reported an increase in β-catenin mRNA in the livers of FXR-KO mice at 3 months but did not observe changes in protein levels, which was attributed to posttranscriptional regulation of β-catenin. Consistent with this observation, our data indicate no change in β-catenin activation at 3 months of age. However, a significant increase in β-catenin activation was noted at 8 months of age in the livers of FXR-KO mice, which further increased at 14 months. The lack of early increases in β-catenin activation suggests that the mechanisms of β-catenin activation in the FXR-KO mice may be independent of the loss of FXR in the FXR-KO mice.
One of the plausible mechanisms behind β-catenin activation is increased bile acids in the FXR-KO mice. FXR-KO mice are known to have 4-fold higher total bile acids, but the bile acid composition remains largely unchanged (Kim et al., 2007a,b). However, decreasing bile acids using cholestyramine reduces tumor incidence (Yang et al., 2007). It was also observed that long-term bile acid treatment significantly increased dimethylnitrosamine-induced HCC in C57BL/6 mice (Yang et al., 2007). It is interesting to note that hepatocyte-specific FXR-KO mice, which do not have increased bile acids, do not develop HCC (U. Apte, unpublished observations). In addition, the BSEP knockout mice, which have increased bile acids, also develop HCC. Furthermore, children with BSEP mutations develop progressive familial intrahepatic cholestasis and are highly susceptible to the development of HCC (Knisely et al., 2006). These observations indicate that increased bile acids play a critical role in hepatic tumorigenesis in the FXR-KO mice. Our data suggest the possibility that increased bile acids may stimulate temporal activation of the Wnt/β-catenin pathway independent of FXR and promote HCC development in FXR-KO mice. However, the exact mechanisms by which bile acids induce β-catenin are not known currently. Bile acids are known to initiate FXR-independent signaling via epidermal growth factor receptor and extracellular signal-regulated kinase 1/2 signaling pathways (Hylemon et al., 2009; Allen et al., 2010). Both extracellular signal-regulated kinase 1/2 and Akt signaling pathways are known to interact with Wnt signaling, resulting in β-catenin activation (Hu and Li, 2010). These observations support the hypothesis that increased bile acids in the FXR-KO mice may stimulate β-catenin activation via mitogen-activated protein kinase signaling.
The molecular mechanisms of β-catenin activation in the FXR-KO mice seem to be multifold. Our data indicate that both the activation of the canonical Wnt signaling pathway and the loss of E-cadherin are involved in β-catenin activation in the livers of FXR-KO mice. Increased Wnt4 and several Fzl receptors, induced Dvl expression, striking inactivation of GSK-3β, and decreased Ser45-Thr41-phosphorylated β-catenin indicate the activation of canonical Wnt signaling in the livers of FXR-KO mice. However, we cannot exclude the possibility that the inactivation of GSK-3β could be independent of canonical Wnt signaling. Studies have shown that mitogen-activated protein kinase signaling can cross-activate β-catenin activation via the inactivation of GSK-3β (Ding et al., 2005; Hu and Li, 2010). Likewise, it is known that insulin signaling induces GSK-3β inactivation, thus converging the insulin signaling and Wnt signaling pathways (Pearl and Barford, 2002). Recent evidence suggests that bile acids can activate Akt, epidermal growth factor receptor, extracellular signal-regulated kinase 1/2, and insulin signaling (Hylemon et al., 2009; Trauner et al., 2010). It is plausible that the GSK-3β inactivation observed in the FXR-KO mice is due to increased bile acids in these mice. These studies point to the possibility that the GSK-3β inactivation observed in the FXR-KO mice may be independent of the canonical Wnt signaling.
E-cadherin plays an important role in the regulation of β-catenin activity in cells by sequestering β-catenin at the cell membrane (Apte et al., 2006; Thompson and Monga, 2007). An increase in β-catenin gene expression results in a concomitant increase in E-cadherin expression, effectively reducing the activity of β-catenin (Apte et al., 2006). The loss of E-cadherin is a hallmark of many cancers, resulting in increased cytoplasmic stabilization of β-catenin (Behrens et al., 1993; Nuruki et al., 1998; Calvisi et al., 2004b; Jeanes et al., 2008). We observed a marked decrease in E-cadherin expression in the livers of FXR-KO mice, which is consistent with increased β-catenin activation. A well known mechanism of inhibited E-cadherin expression is gene repression by the transcriptional repressor Snail, which occurs during the epithelial-to-mesenchymal transition and is well documented during carcinogenesis (Batlle et al., 2000; Cano et al., 2000; Huber et al., 2005). Furthermore, previous in vitro studies indicate that bile acids can induce Snail activation, resulting in E-cadherin suppression (Fukase et al., 2008). Consistent with these observations, we observed an increase in Snail activation in the FXR-KO mice. These data indicate that epithelial-to-mesenchymal transition-dependent repression of E-cadherin resulting in subsequent β-catenin activation may be involved in the pathogenesis of HCC in FXR-KO mice. The molecular mechanism of decreased E-cadherin expression in the FXR-KO mice is not completely clear. It is possible that increased bile acids in FXR-KO mice induce Snail expression, which suppresses E-cadherin expression. However, recent chromatin immunoprecipitation sequencing studies on the FXR binding site in liver and intestine indicate that FXR has multiple binding sites upstream of the E-cadherin gene in the liver (Thomas et al., 2010). This observation suggests that the loss of E-cadherin expression in the FXR-KO mice may be a direct result of FXR deletion. These studies also warrant further analysis of the FXR binding sites on the E-cadherin gene.
To examine whether the observation that the loss of FXR results in increased HCC development is relevant for human HCC pathogenesis, we studied whether FXR expression and function decrease in human HCC. Our data indicate decreased FXR mRNA and activity (as demonstrated by decreased expression of SHP, a target of FXR) as early as stage I of HCC. Whereas these data do not provide evidence for the loss of FXR during HCC pathogenesis, they do indicate that the loss of FXR function is associated with HCC and thus may be involved in HCC development. A detailed analysis of FXR expression and function and bile acid levels during the various stages of HCC pathogenesis, including steatohepatitis, fibrosis, and cirrhosis, is required for further understanding the role of FXR and bile acids in the pathogenesis of HCC, but such an analysis is beyond the scope of this article.
Taken together, these data provide strong evidence for increased Wnt/β-catenin pathway activation during HCC development in FXR-KO mice. These observations also provide a basis for the hypothesis that increased bile acids promote HCC development via stimulation of Wnt signaling. Whereas further studies using tissue-specific double-knockout mice and the modulation of total bile acid levels are required to test this hypothesis, the present observations support a role for β-catenin activation during the pathogenesis of HCC in the FXR-KO mice. These data indicate that a detailed analysis of FXR, bile acids, and Wnt signaling will reveal novel therapeutic targets for HCC treatment.
Supplementary Material
Acknowledgments
We thank Guodong Li and Marimaran Rengasamy for technical support.
This work was supported by the National Institutes of Health National Center for Research Resources [Grant 1 P20-RR021940-03]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK031343]; and University of Kansas Endowment Startup Funds.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.179390.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- FXR
- farnesoid X receptor
- HCC
- hepatocellular carcinoma
- GSK-3β
- glycogen synthase kinase 3β
- Dvl
- dishevelled
- Fzl
- frizzled
- FXR-KO
- farnesoid X receptor knockout
- SHP
- small heterodimer partner
- BSEP
- bile salt export pump
- PCR
- polymerase chain reaction
- WT
- wild-type
- RIPA
- radioimmunoprecipitation assay.
Authorship Contributions
Participated in research design: Wolfe, Guo, and Apte.
Conducted experiments: Wolfe, Thomas, Edwards, and Jaseja.
Performed data analysis: Wolfe, Thomas, Guo, and Apte.
Wrote or contributed to the writing of the manuscript: Guo and Apte.
References
- Allen K, Kim ND, Moon JO, Copple BL. (2010) Upregulation of early growth response factor-1 by bile acids requires mitogen-activated protein kinase signaling. Toxicol Appl Pharmacol 243:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. (2009) Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol 175:1056–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte U, Zeng G, Muller P, Tan X, Micsenyi A, Cieply B, Dai C, Liu Y, Kaestner KH, Monga SP. (2006) Activation of Wnt/beta-catenin pathway during hepatocyte growth factor-induced hepatomegaly in mice. Hepatology 44:992–1002 [DOI] [PubMed] [Google Scholar]
- Aravalli RN, Steer CJ, Cressman EN. (2008) Molecular mechanisms of hepatocellular carcinoma. Hepatology 48:2047–2063 [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2:84–89 [DOI] [PubMed] [Google Scholar]
- Befeler AS, Di Bisceglie AM. (2002) Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 122:1609–1619 [DOI] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. (1993) Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 120:757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvisi DF, Factor VM, Ladu S, Conner EA, Thorgeirsson SS. (2004a) Disruption of beta-catenin pathway or genomic instability define two distinct categories of liver cancer in transgenic mice. Gastroenterology 126:1374–1386 [DOI] [PubMed] [Google Scholar]
- Calvisi DF, Ladu S, Conner EA, Factor VM, Thorgeirsson SS. (2004b) Disregulation of E-cadherin in transgenic mouse models of liver cancer. Lab Invest 84:1137–1147 [DOI] [PubMed] [Google Scholar]
- Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2:76–83 [DOI] [PubMed] [Google Scholar]
- Carruba G, Cervello M, Miceli MD, Farruggio R, Notarbartolo M, Virruso L, Giannitrapani L, Gambino R, Montalto G, Castagnetta L. (1999) Truncated form of beta-catenin and reduced expression of wild-type catenins feature HepG2 human liver cancer cells. Ann NY Acad Sci 886:212–216 [DOI] [PubMed] [Google Scholar]
- Devereux TR, Stern MC, Flake GP, Yu MC, Zhang ZQ, London SJ, Taylor JA. (2001) CTNNB1 mutations and beta-catenin protein accumulation in human hepatocellular carcinomas associated with high exposure to aflatoxin B1. Mol Carcinog 31:68–73 [DOI] [PubMed] [Google Scholar]
- Di Bisceglie AM, Befeler AS. (2007) Diagnostic and therapeutic approach to hepatocellular carcinoma in the USA. Hepatol Res 37 (Suppl 2):S251–S253 [DOI] [PubMed] [Google Scholar]
- Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, et al. (2005) Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell 19:159–170 [DOI] [PubMed] [Google Scholar]
- Eloranta JJ, Kullak-Ublick GA. (2008) The role of FXR in disorders of bile acid homeostasis. Physiology (Bethesda) 23:286–295 [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Mason AC. (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340:745–750 [DOI] [PubMed] [Google Scholar]
- Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, et al. (1995) Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81:687–693 [DOI] [PubMed] [Google Scholar]
- Fujie H, Moriya K, Shintani Y, Tsutsumi T, Takayama T, Makuuchi M, Kimura S, Koike K. (2001) Frequent beta-catenin aberration in human hepatocellular carcinoma. Hepatol Res 20:39–51 [DOI] [PubMed] [Google Scholar]
- Fukase K, Ohtsuka H, Onogawa T, Oshio H, Ii T, Mutoh M, Katayose Y, Rikiyama T, Oikawa M, Motoi F, et al. (2008) Bile acids repress E-cadherin through the induction of Snail and increase cancer invasiveness in human hepatobiliary carcinoma. Cancer Sci 99:1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ. (2010) Bile acids and their nuclear receptor FXR: relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta 1801:683–692 [DOI] [PubMed] [Google Scholar]
- Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Jr, Kliewer SA, Gonzalez FJ, Sinal CJ. (2003) Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem 278:45062–45071 [DOI] [PubMed] [Google Scholar]
- Hu T, Li C. (2010) Convergence between Wnt-β-catenin and EGFR signaling in cancer. Mol Cancer 9:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. (1999) Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol 155:1795–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD. (2006) Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312:233–236 [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558 [DOI] [PubMed] [Google Scholar]
- Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. (2009) Bile acids as regulatory molecules. J Lipid Res 50:1509–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes A, Gottardi CJ, Yap AS. (2008) Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene 27:6920–6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. (2007a) Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48:2664–2672 [DOI] [PubMed] [Google Scholar]
- Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. (2007b) Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28:940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikçi B, Ozçay F, et al. (2006) Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44:478–486 [DOI] [PubMed] [Google Scholar]
- Kolligs FT, Bommer G, Göke B. (2002) Wnt/beta-catenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis. Digestion 66:131–144 [DOI] [PubMed] [Google Scholar]
- Lambert G, Amar MJ, Guo G, Brewer HB, Jr, Gonzalez FJ, Sinal CJ. (2003) The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem 278:2563–2570 [DOI] [PubMed] [Google Scholar]
- Llovet JM, Bruix J. (2008) Molecular targeted therapies in hepatocellular carcinoma. Hepatology 48:1312–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran RR, Thomas A, Roth M, Sheng Z, Esterly N, Pinson D, Gao X, Zhang Y, Ganapathy V, Gonzalez FJ, et al. (2009) Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther 328:469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. (2008) Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res 68:9589–9594 [DOI] [PubMed] [Google Scholar]
- Monga SP, Micsenyi A, Germinaro M, Apte U, Bell A. (2006) β-Catenin regulation during matrigel-induced rat hepatocyte differentiation. Cell Tissue Res 323:71–79 [DOI] [PubMed] [Google Scholar]
- Monga SP, Monga HK, Tan X, Mulé K, Pediaditakis P, Michalopoulos GK. (2003) Beta-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology 124:202–216 [DOI] [PubMed] [Google Scholar]
- Nuruki K, Toyoyama H, Ueno S, Hamanoue M, Tanabe G, Aikou T, Ozawa M. (1998) E-cadherin but not N-cadherin expression is correlated with the intracellular distribution of catenins in human hepatocellular carcinomas. Oncol Rep 5:1109–1114 [DOI] [PubMed] [Google Scholar]
- Pearl LH, Barford D. (2002) Regulation of protein kinases in insulin, growth factor and Wnt signalling. Curr Opin Struct Biol 12:761–767 [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. (2000) Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744 [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, Nagorney DM, Burgart LJ, Roche PC, Smith DI, et al. (2002) Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 21:4863–4871 [DOI] [PubMed] [Google Scholar]
- Thomas AM, Hart SN, Kong B, Fang J, Zhong XB, Guo GL. (2010) Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology 51:1410–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MD, Monga SP. (2007) WNT/beta-catenin signaling in liver health and disease. Hepatology 45:1298–1305 [DOI] [PubMed] [Google Scholar]
- Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. (2010) Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis 28:220–224 [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Moore DD, Huang W. (2008) FXR: a metabolic regulator and cell protector. Cell Res 18:1087–1095 [DOI] [PubMed] [Google Scholar]
- Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. (2007) Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res 67:863–867 [DOI] [PubMed] [Google Scholar]
- Zeng G, Awan F, Otruba W, Muller P, Apte U, Tan X, Gandhi C, Demetris AJ, Monga SP. (2007) Wnt'er in liver: expression of Wnt and frizzled genes in mouse. Hepatology 45:195–204 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.