Abstract
Background
Increased understanding of the pathophysiology of the acute coagulopathy of trauma has lead many to question the current transfusion approach to hemorrhagic shock. We hypothesized that warm fresh whole blood (WFWB) transfusion would be associated with improved survival in patients with trauma compared with those transfused only stored component therapy (CT).
Methods
We retrospectively studied US Military combat casualty patients transfused ≥1 unit of red blood cells (RBCs). The following two groups of patients were compared: (1) WFWB, who were transfused WFWB, RBCs, and plasma but not apheresis platelets and (2) CT, who were transfused RBC, plasma, and apheresis platelets but not WFWB. The primary outcomes were 24-hour and 30-day survival.
Results
Of 354 patients analyzed there were 100 in the WFWB and 254 in the CT group. Patients in both groups had similar severity of injury determined by admission eye, verbal, and motor Glasgow Coma Score, base deficit, international normalized ratio, hemoglobin, systolic blood pressure, and injury severity score. Both 24-hour and 30-day survival were higher in the WFWB cohort compared with CT patients, 96 of 100 (96%) versus 223 of 254 (88%), (p = 0.018) and 95% to 82%, (p = 0.002), respectively. An increased amount (825 mL) of additives and anticoagulants were administered to the CT compared with the WFWB group, (p < 0.001). Upon multivariate logistic regression the use of WFWB and the volume of WFWB transfused was independently associated with improved 30-day survival.
Conclusions
In patients with trauma with hemorrhagic shock, resuscitation strategies that include WFWB may improve 30-day survival, and may be a result of less anticoagulants and additives with WFWB use in this population.
Keywords: Whole blood, Transfusion, Mortality, Survival, Combat
Traumatic injury is the leading cause of death for patients between the ages of 1 to 40 years.1 Approximately 150,000 people die per year in the United States from traumatic injuries.2 US Military reports estimate that 15% to 20% of traumatic deaths are preventable, and 66% to 80% of these deaths occur from hemorrhage.3,4 Rural civilian data indicates that approximately 10% of deaths are preventable.5,6 If 10% to 20% of 150,000 US civilian traumatic deaths are preventable, and 66% to 80% of these preventable deaths are due to uncontrolled bleeding this translates to between 10,000 and 24,000 potentially preventable hemorrhagic trauma deaths per year in the United States. Hemorrhagic deaths typically occur within the first 24 hours of admission.7-9 Early identification and prevention of patients who are at risk of developing coagulopathy and subsequent strategies to control coagulopathic bleeding may therefore improve survival.10-13
The transfusion approach to hemorrhagic injuries has evolved or continually progressed since it developed in the early 1900s. The evolution has included whole blood, modified whole blood to the current use of component therapy (CT).14,15 After the development of whole blood fractionation, CT now predominates as the primary transfusion approach secondary to concerns for resource utilization and safety.16-18 This change occurred without evidence comparing the benefits or risks between these products specifically for patients with traumatic hemorrhagic shock.16-18 Classic transfusion guidelines regarding indications for blood components are based on expert opinion, experiments in euvolemic patients requiring elective surgery, and modified whole blood that is no longer commonly available.16-18 In addition, the storage age of red blood cells (RBCs) has progressively increased over time to a current limit of 42 days14,15 without prospective study evaluating the clinical effect of increased RBC storage length in critically ill patients.
Recently, there has been a change in opinion by some in the trauma community regarding the transfusion approach to hemorrhagic shock. The concept of hemostatic or damage control resuscitation as the optimal approach for the treatment of patients with severe life-threatening hemorrhagic traumatic injuries is gaining wide acceptance.19 This approach advocates for the rapid control of surgical bleeding, transfusion of RBCs, plasma, and platelets in a 1:1:1 ratio; preference for the use of fresh RBCs; limitation of excessive crystalloid use; and prevention of acidosis and hypothermia.20-25 The use of warm fresh whole blood (WFWB) has also been utilized in US Military facilities out of necessity at combat hospitals in Afghanistan and Iraq to support the application of damage control resuscitation principles.18,26-28 A recent review describes over 6000 units of WFWB that has been transfused in recent combat operations.29 The recent large scale use of WFWB by the US Military has rekindled interest of its use for patients at high risk of death from hemorrhage to include civilian disaster emergencies.29
Theoretically, WFWB may be advantageous for patients in hemorrhagic shock over CT due to improved function of RBCs, plasma, and platelets, and avoidance of the adverse effects of the storage lesion when older RBCs are transfused. Interestingly, comparisons between patients transfused WFWB to those receiving only stored CT for traumatic hemorrhagic shock has not been evaluated. We hypothesized that WFWB transfusion would improve both short-term (24 hour) and 30-day survival when compared with patients transfused only stored CT in this population.
METHODS
This retrospective study was approved by the Institutional Review Board at Brooke Army Medical Center, San Antonio, TX. US Military patients were identified from a transfusion database maintained at the US Army Institute of Surgical Research. The database includes patients injured in both Afghanistan and Iraq and transfused at least one unit of RBCs between January 2004 and October 2007 at either a level II or a level III hospital. The following two groups of patients were compared: (1) WFWB, who were transfused WFWB, RBCs, and plasma but not apheresis platelets (aPLT) and (2) CT, who were transfused RBC, plasma, and aPLT but not WFWB. Patients were excluded if they received both WFWB and aPLTs or if they were transfused neither WFWB nor aPLTs. The primary outcomes were 24-hour and 30-day survival. All blood product amounts were measured at 24 hours from admission. The following terms are used in the article to describe different products. Stored RBCs are red cells that are stored in AS-5 solution that are shipped from the United States. The stored RBCs transfused to patients in this study were neither leukoreduced before storage nor were they at the time of transfusion. Total RBC units were calculated by adding RBC units and one unit of RBCs from each WFWB unit transfused. Plasma indicates both fresh-frozen plasma and thawed plasma. Apheresis platelets were collected at combat support hospitals and were stored up to 7 days. The total plasma to RBC ratio was calculated by also including a unit of plasma and RBCs from each unit of WFWB transfused, (plasma + WFWB/RBC + WFWB). The total platelet to RBC ratio was calculated by incorporating six units of platelets for each unit of apheresis platelet unit transfused or one unit of platelets for each unit of WFWB (WFWB + 6[aPLT]/[WFWB + RBC). Massive transfusion was defined as 10 or more total RBC units within the first 24 hours of admission. Volumes of each blood product were determined using standard volumes of each product transfused: RBC 360 mL, FFP 270 mL, aPLT 300 mL, and WFWB 450 mL. Anticoagulant or additive solutions within blood products transfused to both groups were determined by adding the following standard amounts for each product: RBC 120 mL, plasma 50 mL, aPLTs 35 mL, and WFWB 63 mL. Actual volume of blood products transfused was calculated by subtracting anticoagulant/additive from the volume of total blood products. Additional variables included in our analysis was patient age, admission vital signs, and laboratory values to include individual eye, verbal and motor Glasgow Coma Score (GCS), temperature (T), systolic blood pressure, heart rate, hemoglobin, base deficit, International Normalized Ratio, and injury severity score (ISS). The ISS was calculated by trained staff at the US Army Institute of Surgical Research according to the methods described by the Association for the Advancement of Automotive Medicine Abbreviated Injury Scale, 1998 Revision.30
To determine whether changes in capabilities of combat support hospitals over time influenced our results we measured 30-day survival for both study groups before and after 1 December 2006. This arbitrary cut off date was chosen to allow for equal amounts of patients who received CT to be represented in the groups compared. Adverse events such as deep vein thrombosis, pulmonary embolism, myocardial infarction, cerebral stroke, acute respiratory distress syndrome, and renal failure were recorded if these events were documented in the patients’ charts. Strict definitions were neither utilized for each of these adverse events nor there was prospective screening for any of these events. The term traumatic hemorrhagic shock in this article is arbitrarily defined by the authors as patients with a base deficit of three or higher secondary to bleeding from traumatic injuries. A base deficit of three is abnormal and very likely represents lactic acidosis, anaerobic metabolism, or mild shock when it is measured upon admission in healthy patients who have not received a large amount of fluid resuscitation and present with traumatic injuries. As a result, the term shock as we liberally define it, for the purposes of describing the patients only in this study, ranges from mild to severe or compensated to uncompensated hemorrhagic shock.
Statistical Analysis
Median (interquartile range) was used to describe all data. Wilcoxon’s rank-sum, χ2, and Fisher’s exact tests were used for statistical comparisons as appropriate. GCS values for eye, verbal, and motors were categorized into normal or abnormal responses when included into the multivariate regression model. Normal values for GCS eye, verbal, and motor were defined as 4, 5, and 6, respectively. All variables on univariate analysis with 30-day survival with a p value of <0.1 were considered for inclusion in a multivariate logistic regression analysis with backwards stepwise method to determine independent associations with 30-day survival. Only variables that were independently associated with 30-day survival are reported, and those that were not significant are not described as a result of using a backwards stepwise elimination method. Statistical analysis was performed with (SPSS, 15.0, Chicago, IL).
RESULTS
In the database, 968 patients were transfused ≥1 U RBC and 354 (37%) met criteria for inclusion into our analysis. There were 100 of 354 (28%) patients in the WFWB group and 254 of 354 (72%) in the CT group. For both groups median (interquartile range) ISS was 18 (10–26) and 30-day survival was 304 of 354 (86%). Patients receiving WFWB (n = 100) compared with CT (n = 254) had similar severity of injury determined by admission base deficit 6 (4–10) to 6 (3–11), International Normalized Ratio 1.4 (1.1–1.6) to 1.4 (1.2–1.8), and ISS 18 (10–26) to 18 (10–26), respectively, (p > 0.05), in addition to similar admission hemoglobin and systolic blood pressure (Table 1). The only difference between the study groups for admission vital signs and laboratory results was that the WFWB group did have decreased admission temperature compared with the CT group, 97.6 (96.4–98.2) versus 98.5 (97.4–99.5) and p ≤ 0.001.
Table 1.
Comparison of Variables Between WFWB and CT Groups
| Variable | WFWB (n = 100) | CT (n = 254) | p Value |
|---|---|---|---|
| Age (yr) | 24 (21–29) | 23 (21–28) | 0.16 |
| Temperature (F) | 97.6 (96.4–98.2) | 98.5 (97.4–99.5) | <0.001 |
| Heart rate (bpm) | 112 (95–136) | 115 (91–138) | 0.88 |
| SBP (mm Hg) | 110 (80–122) | 109 (80–130) | 0.67 |
| GCS eye | 4 (2–4) | 4 (1–4) | 0.32 |
| GCS verbal | 5 (1–5) | 5 (1–5) | 0.53 |
| GCS motor | 6 (3–6) | 6 (1–6) | 0.19 |
| Hemoglobin (g/dL) | 11.6 (10–14) | 11.8 (9.8–13.4) | 0.44 |
| Base deficit | 6 (4–10) | 6 (3–11) | 0.77 |
| INR | 1.4 (1.1–1.6) | 1.4 (1.2–1.8) | 0.83 |
| ISS | 18 (10–26) | 18 (10–26) | 0.74 |
Data presented as Median (IQR) or as percentages
SBP, systolic blood pressure; INR, International Normalized Ratio.
In the WFWB group, the median percentage of total volume of WFWB transfused per total blood products was 2.25 of 7.4 (L), (30%). The individual amounts of each blood product transfused at 24 hours were each different, which is expected because groups were determined by the blood products transfused (Table 2). The transfusion approach was similar with respect to the total RBCs transfused, 16 (11–22) versus 16 (10–22) (p = 0.44), percentage of patients receiving recombinant activated factor VII, 42 versus 40%, (p = 0.72), and the median ratios of plasma to RBCs transfused between WFWB and CT study groups, 0.73 (0.53–1) versus 0.74 (0.55–0.9), (p = 0.73), respectively, (Table 2). Differences in transfusion approach between the groups were the median ratio of platelets to RBCs was decreased in the WFWB compared with the CT group 0.33 (0.2–05) versus 0.86 (0.6–1.3), respectively, (p = 0.001), and the incidence of massive transfusion was increased in the WFWB group compared with the CT group, 89% versus 78%, respectively, (p = 0.017; Table 2). Total, actual, and anticoagulant or additive volumes were all increased in the CT compared with the WFWB group. The net median increase of anticoagulants and additives administered to the CT group was 825 mL in the first 24 hours of admission (Table 2).
Table 2.
Comparison of Individual Blood Products, Volumes and Ratios Between WFWB and CT Groups
| Variable | WFWB (n = 100) | CT (n = 254) | p Value |
|---|---|---|---|
| Stored RBC (U) | 9 (7–14) | 16 (10–22) | <0.001 |
| Plasma (U) | 4 (3–8) | 10 (6–16) | <0.001 |
| Apheresis platelets (U) | 0 | 2 (1–4) | <0.001 |
| WFWB (U) | 5 (3–9) | 0 (0–0) | <0.001 |
| Cryoprecipitate (U) | 0 (0–0) | 0 (0–1) | 0.007 |
| Total RBC (U) | 16 (11–22) | 16 (10–22) | 0.44 |
| Total blood volume (L) | 7.4 (5.4–10.4) | 9.3 (6.2–13.3) | 0.006 |
| Anticoagulant/ additives (L) |
1.7 (1.3–2.5) | 2.5 (1.6–3.6) | <0.001 |
| Actual blood volume (L) | 5.7 (4.1–8.) | 6.8 (4.5–10) | 0.03 |
| PLT:RBC ratio | 0.33 (0.2–0.5) | 0.86 (0.6–1.3) | 0.001 |
| Plasma:RBC ratio | 0.74 (0.55–0.9) | 0.73 (0.53–1) | 0.73 |
| Massive transfusion (%) |
89/100 (89%) | 198/254 (78%) | 0.017 |
| rFVIIa use (%) | 42/100 (42%) | 101/353 (40%) | 0.72 |
Data presented as Median (IQR) or as percentages.
rFVIIa, recombinant factor VIIa.
Variables that were associated with 30-day survival are described in Tables 3 and 4. In addition to the typical admission vital signs and laboratory values that are associated with survival, our results indicate that the amount of WFWB, plasma: RBC ratio, and less anticoagulant and additive volume were each associated with increased survival on univariate analysis. The patient group transfused WFWB had improved 24 hour, 96 of 100 (96%) versus 223 of 254 (88%), (p = 0.018), and 30-day survival 95 of 100 (95%) to 209 of 254 (82%), (p = 0.002), compared with the CT group, respectively, (Table 5). The Kaplan-Meier Curve for 30-day survival also indicates that 30-day survival was increased for patients in the WFWB group (p = 0.002; Fig. 1). Comparison of all adverse events recorded indicated that renal failure was more frequent in the WFWB compared with the CT group, 8% versus 3%, respectively, (p = 0.04). An increased incidence of deep vein thrombosis and acute respiratory distress syndrome approached significance (Table 5).
Table 3.
Comparison of Variables for Survivors and Nonsurvivors at 30 d
| Variable | Dead (n = 50) | Alive (n = 304) | p Value |
|---|---|---|---|
| Age (yr) | 23.5 (21–29.3) | 23 (21–27) | 0.85 |
| Temperature (F) | 97.5 (94.2–99.3) | 98.1 (97.3–99.2) | 0.17 |
| Heart rate (bpm) | 108 (60–135) | 106 (87–129) | 0.21 |
| SBP (mm Hg) | 102 (53–133) | 110 (83–129) | 0.16 |
| Hemoglobin (g/dL) | 9.7 (7.4–12.4) | 11.9 (10.1–13.5) | 0.001 |
| GCS eye | 1 (1–3) | 4 (3–4) | <0.001 |
| GCS verbal | 1 (1–4) | 5 (1–5) | <0.001 |
| GCS motor | 1 (1–4) | 6 (4–6) | <0.001 |
| Base deficit | 14 (6–20) | 5 (3–9) | <0.001 |
| INR | 1.8 (1.3–2.7) | 1.4 (1.1–1.6) | <0.001 |
| ISS | 25 (18–33) | 17 (10–25) | <0.001 |
Data presented as Median (IQR) or as percentages.
SBP, systolic blood pressure; INR, International Normalized Ratio.
Table 4.
Univariate Comparison of Blood Products, Agents and Ratios for Survivors and Nonsurvivors at 30 d
| Variable | Dead (n = 50) | Alive (n = 304) | p Value |
|---|---|---|---|
| Stored RBC (U) | 19 (11–24) | 12 (8–19) | 0.001 |
| Plasma (U) | 10 (5–16) | 9 (5–14) | 0.46 |
| Apheresis platelets (U) | 2 (1–4) | 1 (0–3) | 0.01 |
| WFWB (U) | 0 (0–0) | 0 (0–3) | 0.002 |
| Cryoprecipitate (U) | 0 (0–1) | 0 (0–1) | 0.74 |
| Massive transfusion % | 43/50 (86%) | 244/304 (80%) | 0.44 |
| rFVIIa use (%) | 29/50 (58%) | 114/304 (38%) | 0.007 |
| Plasma:RBC | 0.58 (0.4–0.89) | 0.75 (0.56–0.95) | 0.003 |
| Platelet:RBC | 0.69 (0.4–1.1) | 0.66 (0.44–1.1) | 0.92 |
| Actual blood volume (L) | 7.2 (4.8–10.7) | 6.3 (4.8–9.1) | 0.1 |
| Anticoagulant/ additive volume (L) |
2.9 (1.7–3.9) | 2.2 (1.4–3.2) | 0.03 |
Data presented as Median (IQR) or as percentages.
rFVIIa, Recombinant Factor VIIa.
Table 5.
Comparison of Survival Outcomes and Adverse Events Between Study Groups
| Variable | WFWB (n = 100) | CT (n = 254) | p Value |
|---|---|---|---|
| 24 h survival | 96/100 (96%) | 223/254 (88%) | 0.018 |
| 30 d survival | 95/100 (95%) | 209/254 (82%) | 0.002 |
| Deep vein thrombosis |
15/100 (15%) | 21/254 (8%) | 0.06 |
| Pulmonary embolism | 7/100 (7%) | 11/254 (4%) | 0.3 |
| Myocardial infarction | 1/100 (1%) | 0 (0%) | 0.28 |
| Cerebral stroke | 0 (0%) | 5/254 (2%) | 0.33 |
| ARDS | 7/100 (7%) | 7/254 (3%) | 0.08 |
| Renal failure | 8/100 (8%) | 7/254 (3%) | 0.04 |
ARDS, Acute Respiratory Distress Syndrome.
Fig. 1.
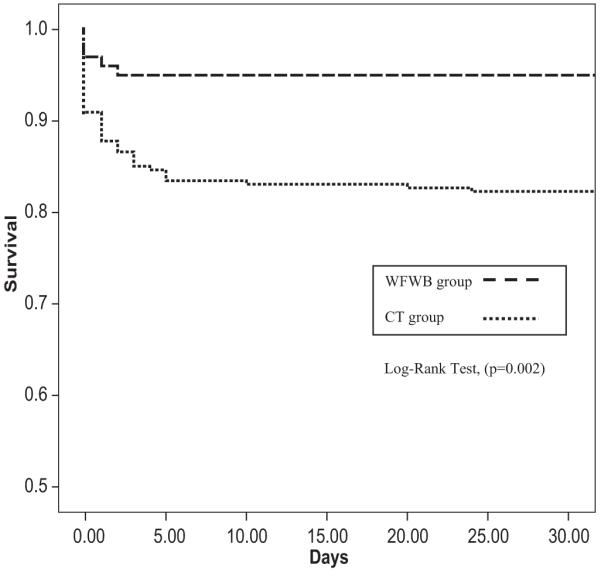
Kaplan-Meier curve of 30-day survival according to study group.
Multivariate logistic regression revealed that the group of patients transfused WFWB, (OR 12.4 [1.8–80], [p = 0.01]) and an increased plasma: RBC ratio (OR 11.7 [2.6–52], p = 0.001) were both independently associated with improved 30-day survival, (Table 6). The multivariate logistic regression analysis utilizing individual blood product amounts indicated that each unit of WFWB (OR 2.15 [1.21–3.8], [p = 0.016]) and plasma (OR 1.09 [1.02–1.18], [p = 0.019]) was independently associated with improved 30-day survival and each unit of RBCs (OR [0.91[, [p = 0.003]) was independently associated with decreased 30-day survival. The amount of apheresis platelets was not independently associated with 30-day survival after adjusting for confounding variables in our best-fit model (Table 7).
Table 6.
Multivariate Logistic Regression With Treatment Groups for 30-d Survival
| Variables | OR (95.0% C.I.) | p Value |
|---|---|---|
| WFWB group | 12.4 (1.8–80) | 0.01 |
| Plasma:RBC ratio | 11.7 (2.6–52) | 0.001 |
| ISS | 0.94 (0.91–0.97) | 0.001 |
| GCS eyes (normal) | 4.1 (1.5–10.8) | 0.004 |
| Base deficit | 0.88 (0.82–0.95) | <0.001 |
AUC (95% CI) for the logistic regression was 0.9 (0.85–0.95).
Table 7.
Multivariate Logistic Regression Results With Blood Product Amount for 30-d Survival
| Variables | OR (95.0% C.I.) | p Value |
|---|---|---|
| WFWB (U) | 2.15 (1.21–3.8) | 0.016 |
| RBC (U) | 0.91 (0.85–0.97) | 0.003 |
| Plasma (U) | 1.09 (1.02–1.18) | 0.019 |
| Base deficit | 0.91 (0.84–0.97) | 0.002 |
| GCS eyes (normal) | 3.8 (1.4–10.2) | 0.009 |
| ISS | 0.94 (0.91–0.98) | 0.001 |
AUC (95% CI) for the logistic regression was 0.9 (0.86–0.95).
To determine whether changes in capabilities of combat support hospitals over time influenced our results we measured 30-day survival for both study groups before and after December 1, 2006. Before this date survival in the WFWB and CT groups were 67 of 71 (94.4%) versus 102 of 126 (81%), respectively, (p = 0.01). After December 1, 2006 survival in the WFWB and CT groups were 28 of 29 (96.6%) versus 107 of 128 (83.6%), respectively, (p = 0.08).
DISCUSSION
This large retrospective study is unique in that it is the first to report improved 24-hour and 30-day survival for patients transfused WFWB with traumatic hemorrhagic shock when compared with patients transfused CT with similar severity of injury. A 13% increase in 30-day survival was measured with the use of WFWB in combat casualties transfused one or more units of RBCs compared with the CT group. It is also the first to indicate that the volume of WFWB transfused is independently associated with improved 30-day survival. The main difference between the groups that may have contributed to our results was that the patients in the CT group received an increased amount of anticoagulants and additives, and an increased amount of stored RBCs in the first 24 hours. In a subset analysis, our data indicates that as time progressed and capabilities improved at combat support hospitals that the relationship between improved survival and WFWB use remained and was not influenced by this factor.
Our results confirm previous studies that indicate that increased ratios of plasma to RBCs and increased volume of plasma are independently associated with improved survival in patients with acute coagulopathy of trauma.22,31-34 The findings in our report also compliment previously reported data that the amount of RBCs transfused is independently associated with decreased survival in critically ill patients. The potential mechanisms to explain the adverse effects with the transfusion of stored RBCs of advanced storage age have been summarized in multiple review articles.35-38 In addition, our results are the second to establish that in the same cohort opposite independent effects on survival can be measured by different blood products (RBCs were decreased compared with WFWB and plasma being increased). These results further support the claim that it is possible to adequately adjust for injury severity and obtain accurate results regarding the independent effects of individual blood products.32
The difference in total platelet ratios compared between groups is difficult to interpret since the ratios were calculated according to the amount of platelet units in either WFWB or apheresis platelets compared with one unit of random donor platelets as is described in the methods. The fact that the function of any fresh component with its corresponding stored component is different makes any comparison of volume of that component difficult to analyze and interpret. Despite these limitations, the WFWB group of patients received a decreased ratio of PLT: RBCs, and therefore did not seem to have received a superior transfusion approach that would confound our results. According to our calculation of the total PLT: RBC ratio, patients in the WFWB group received a suboptimal transfusion strategy compared with the CT group. A recent study in patients with trauma indicates that an increased platelet to RBC ratio of above 1:2 was associated with improved 30-day survival,31 and an unpublished report (presented at ATACCC 2008) by Perkins also indicates that patients transfused increased ratios of PLT: RBC with combat-related injuries is also independently associated with improved survival.
There are no previous reports in the literature comparing outcomes for patients transfused WFWB in any population because it is almost exclusively used in combat operations. Whole blood stored cold for less than 48 hours has been compared with CT in neonatal cardiac surgery populations with conflicting results which may be a result of methodological differences and inclusion criteria between these studies.39,40
In this retrospective study from many combat support hospitals, we were not able to collect data that would allow us to precisely determine the mechanisms in which WFWB might improve survival. The patients included in our study who received more than one unit of RBCs ranged from presenting in compensated to uncompensated hemorrhagic shock according to admission vital signs and laboratory values. It is our belief that WFWB is more efficient than stored CT at correcting coagulopathy and shock in this population and also minimizes the adverse effects of the transfusion of the storage lesion of older RBCs.35-38 There are multiple previously published reports that review the mechanisms in which WFWB would improve survival compared with stored CT for patients in hemorrhagic shock. Fresh whole blood or fresh RBCs improves cardiac output, microcirculatory hemodynamics, and oxygen consumption compared with older stored whole blood or RBCs.41-45 Each of these findings support improved function of RBCs when transfused fresh which improves their ability to prevent or correct shock or oxygen debt in critically ill patients. WFWB is also a more concentrated and functional product when compared with stored components reconstituted in a 1:1:1 ratio to reflect whole blood.46 When components are reconstituted after the addition of anti-coagulants and additive solutions a cold and dilute anemic and hypocoaguable product is produced. For patients with the acute coagulopathy of trauma, WFWB transfusion may correct coagulopathy more efficiently than stored components as a result of the increased function and concentration of platelets and plasma when compared with stored components.46-48
An additional potential mechanism of the increased mortality measured in the CT group was the increased volume of anticoagulants and additives transfused to this group. The additional median 825 mL of anticoagulants and additives in the first 24 hours of admission may have increased the risk of both dilutional coagulopathy and significant anticoagulation in patients at high risk of death from hemorrhage. As future studies are designed it is important that the amount of anti-coagulants and additives be measured and their clinical effects determined. With increased amounts of component products transfused the amount of anticoagulants and additives will increase and may potentially adversely affect patients in hemorrhagic shock. Based on the standard amounts of anticoagulants listed in the “Methods” section one unit of reconstituted whole blood from one unit each of stored RBCs, plasma, and apheresis platelets will contain 279 mL of anti-coagulants and additives compared with only 63 to 70 mL in one unit of whole blood. It is also interesting to note that the CT group received an increased volume of total and actual blood products in the first 24 hours despite decreased time alive during that time period. These results may have also been influenced by the increased amount of anticoagulants and additives transfused to the CT group, which potentiated bleeding and required more total and actual volume of blood products. Another perspective may be that the more concentrated and functionally efficient WFWB product reversed the acute coagulopathy of trauma better than CT, which resulted in improved survival and permitted decreased use of total blood products.
A third potential mechanism that can explain our results is that WFWB transfusion may improve outcomes in critically ill patients by minimizing the transfusion of RBCs of advanced storage age. Because of logistical constraints in Combat Support Hospitals in Iraq the median storage age of RBCs transfused is 33 days.37 This is not too dissimilar to the average storage age of 21 days reported in the United States.49 The adverse effects of the transfusion of RBCs of advanced storage age have been thoroughly reviewed and include inflammatory injury, impaired vasoregulation, and immune modulation, as well as decreased RBC deformability and increased RBC adhesion and aggregation.37 These effects predominantly occur after 14 to 21 days of storage.36,37 Retrospective studies also indicate increased risk of sepsis, multiorgan failure, and death associated with the transfusion of RBCs of advanced storage age (Spinella, submitted).50-52 The use of WFWB was associated with a significant decrease in the use or need to transfuse stored RBCs of advanced age, which may have contributed to the improved survival measured in this group.
The clinical effects of transfusing functional white blood cells are unknown in this population. There is the potential that the transfusion of increased amounts of WBCs may promote inflammatory injury. Transfusion-associated microchimerism (TA-MC) has been reported to be associated with the transfusion of viable white blood cells in RBC units of decreased storage age in patients with trauma.53 The clinical significance of this phenomenon has not been clearly defined.53 The development of transfusion-associated graft versus host disease or any other immunologic disorder has not been reported in patients with trauma as a result of TA-MC. Transfusion reactions were similar in a recent report comparing combat casualty patients transfused WFWB to those who were not.37 In addition, the use of prestorage leukoreduced RBCs in patients with trauma has not been demonstrated to improve outcomes.54,55 Contrary to the concern that the transfusion of fresh WBCs may be detrimental, it may also be possible that the transfusion of functional WBCs from a healthy volunteer may actually improve modulation of the inflammatory system that is both overactive and underactive after traumatic injury.56 Our results that WFWB was associated with increased incidence of renal failure and approached significance with deep vein thrombosis and acute respiratory distress syndrome could potentially be a result of increased inflammation secondary to the transfusion of large amounts of WBCs. Alternatively, because patients in the WFWB group were alive longer than in the CT group, this increased the opportunity for WFWB patients to develop these complications. These complications were also not prospectively screened for, which make the results more difficult to interpret. Lastly, this association between WFWB and renal failure was not adjusted for confounding variables. We did not perform multivariate logistic regression analysis to determine whether any of these complications were independently associated with the use or the amount of WFWB transfused. A larger data set with increased complications would be required for this analysis.
Additional risks associated with the transfusion of WFWB and apheresis platelets collected at combat hospitals are increased risk of transfusion transmitted infectious diseases. We have reported increased risk of hepatitis C and HTLV in donor samples transfused to combat casualties from over 6000 units of WFWB since 2003.29 The risk of transfusion transmitted diseases (TTD’s) with apheresis platelets collected at combat hospitals has not been documented but is possible since methods to formally test each platelet donor is not possible at combat hospitals. The process of screening, collecting, and transfusing WFWB in combat operations has been well described.18,28 Current methods used to minimize the risk of TTD’s for both WFWB and field collected apheresis platelets include donor screening surveys, rapid screening tests of donated products for HIV, HCV and Hepatitis B, and pre-donation screening of potential donors (when possible) for routinely tested TTD’s. In addition all US Military personnel are tested for HIV and immunized against Hepatitis B prior to deployment.
The assessment of the risks and benefits of the transfusion of both WFWB and stored CT (especially when RBCs of advanced storage age are transfused) need to be balanced with the high risk of mortality for patients with traumatic hemorrhagic shock. This balance is likely altered by the severity of injury or degree of critical illness of the patient. Storage lesion effects of RBC are less likely to have clinical effects in noncritically ill patients. In patients who do not have life-threatening bleeding or who are in shock the risks of WFWB may outweigh any potential benefits. Conversely, for patients with traumatic hemorrhagic shock it is our opinion that the survival benefits of WFWB outweigh its risks in combat settings especially when the alternative CT approach includes the use of old RBCs which have also been demonstrated to have adverse clinical effects to include and independent association with increased multiorgan failure and death in patients with trauma.51,52
As a result of the potential survival benefits of WFWB, we also believe that efforts should continue to improve the safety of the volunteer whole blood donor pool which should include HIV, hepatitis B and C screening for all US Military personnel just before deployment. In addition, the development and deployment of rapid immuno-chromatographic screening tests for all other routinely tested transfusion transmitted infectious diseases is needed to diminish the risk of infection in recipients of WFWB and apheresis platelets. Risk of infectious disease transmission is present for both products because they are both collected at combat hospitals and cannot be formally tested for all transfusion transmitted diseases before transfusion. Future developments and refinements of rapid viral inactivation of blood products also has significant potential to eliminate the infectious risk of transfusing blood products and needs to be aggressively pursued.
Upon initial reflection it may seem that WFWB may not be feasible in large civilian trauma centers. With increased investment in blood bank personnel the availability of WFWB in trauma centers with adjacent blood collection centers is possible.
The recent development of infectious disease screening tests that only require 5 hours, and literature that indicates that WFWB can be maintained at room temperature for 72 hours while retaining its coagulation function improve the feasibility of WFWB availability at large civilian trauma centers.57 Fresh whole blood stored warm up to 24 hours and fully tested for all routine infectious agents is available in this manner nationwide in Israel (U. Martinowitz, personal communication). In fact, a large randomized prospective study is about to start in the United States, which will compare survival for patients with traumatic injuries who are transfused whole blood or CT.
The limitations of our study are primarily due to its retrospective nature. As a result, there is increased risk of selection bias and potentially the inability to measure and adjust for all potential confounding factors. In addition, because of the time required to initiate and collect WFWB, patients in this group did not exclusively receive whole blood, and we were forced to compare patients who received WFWB with RBCs and plasma to a cohort who only received CT therapy (RBCs, plasma, platelets). When the estimated volumes of each product as described in the methods are used, WFWB was approximately 30% of the total volume of the blood products transfused in the WFWB group. It would have been preferable to also include data on the amount of crystalloid and colloid fluids administered as well as individual abbreviated injury severity scores to compare between both groups, but this data were not recorded in the database. Comparison of abbreviated injury severity scores would have allowed us to determine whether anatomic injury for instance to the thorax or head was more severe in one group compared with the other. Although, total ISS and each GCS category indicated that both groups compared had equal overall anatomic severity of injury and level of consciousness on admission. Lastly, a significant limitation of our study is that we were not able to determine the precise mechanism for improved survival with WFWB.
Strengths of this study are that is was multicenter, including patients from all seven combat support hospitals in both Iraq and Afghanistan. Also, we only included US patients, which allowed for 100% follow-up on outcomes. Most importantly the groups compared were similar in indicators of severity of injury to include admission vital signs, GCS sub-categories, laboratory values, ISS, total RBC, and actual blood volumes. The survival benefit measured was consistent for patients during early and late time periods of the study.
CONCLUSION
In patients with trauma who present with hemorrhagic shock, resuscitation strategies that include WFWB and an increased ratio of plasma: RBCs may improve 30-day survival. Prospective trials comparing the use of WFWB to the use of full CT are needed.
Footnotes
The views and opinions expressed in this article are those of the authors and do not reflect the official policy or position of the Army Medical Department, Department of the Army, the Department of Defense, or the United States Government.
Contributor Information
Philip C. Spinella, United States Army Institute of Surgical Research, Fort Sam Houston, Texas; Connecticut Children’s Medical Center, Hartford, Connecticut.
Jeremy G. Perkins, United States Army Institute of Surgical Research, Fort Sam Houston, Texas; Walter Reed Army Medical Center, Bethesda, Maryland.
Kurt W. Grathwohl, Brooke Army Medical Center, Fort Sam Houston, Texas.
Alec C. Beekley, Madigan Army Medical Center , Tacoma, Washington.
REFERENCES
- 1.CDC Deaths: final data for 2004. 2007 Available at: http://www.cdc.gov/nchs/deaths.htm. [PubMed]
- 2.AAST [Accessed February 20, 2009];Trauma Facts. 2008 Available at: http://www.aast.org/TraumaFacts/dynamic.aspx?id=964.
- 3.Bellamy RF. The causes of death in conventional land warfare: implications for combat casualty care research. Mil Med. 1984;149:55–62. [PubMed] [Google Scholar]
- 4.Holcomb JB, Caruso J, McMullin NR, et al. Causes of death in special operations forces on the modern battlefield: 2001–2004. Ann Surg. 2007;245:986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito TJ, Sanddal ND, Hansen JD, Reynolds S. Analysis of preventable trauma deaths and inappropriate trauma care in a rural state. J Trauma. 1995;39:955–962. doi: 10.1097/00005373-199511000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Esposito TJ, Sanddal TL, Reynolds SA, Sanddal ND. Effect of a voluntary trauma system on preventable death and inappropriate care in a rural state. J Trauma. 2003;54:663–669. doi: 10.1097/01.TA.0000058124.78958.6B. discussion 669–670. [DOI] [PubMed] [Google Scholar]
- 7.Demetriades D, Murray J, Charalambides K, et al. Trauma fatalities: time and location of hospital deaths. J Am Coll Surg. 2004;198:20–26. doi: 10.1016/j.jamcollsurg.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Peng R, Chang C, Gilmore D, Bongard F. Epidemiology of immediate and early trauma deaths at an urban Level I trauma center. Am Surg. 1998;64:950–954. [PubMed] [Google Scholar]
- 9.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64:1211–1217. doi: 10.1097/TA.0b013e318169cd3c. discussion 1217. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin DF, Niles SE, Salinas J, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 suppl):S57–S63. doi: 10.1097/TA.0b013e318160a566. discussion S63. [DOI] [PubMed] [Google Scholar]
- 12.Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–1463. doi: 10.1097/TA.0b013e318174e8bc. discussion 1463–1465. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205:541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Hess JR, Thomas MJ. Blood use in war and disaster: lessons from the past century. Transfusion. 2003;43:1622–1633. doi: 10.1046/j.1537-2995.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- 15.Starr D. Perennial. Harper Collins; New York: 2002. Blood. [Google Scholar]
- 16.Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190:479–484. doi: 10.1016/j.amjsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 17.McMullin NR, Holcomb JB, Sondeen J. Hemostatic resuscitation. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine. Springer; New York: 2006. pp. 265–278. [Google Scholar]
- 18.Repine TB, Perkins JG, Kauvar DS, Blackborne L. The use of fresh whole blood in massive transfusion. J Trauma. 2006;60(6 suppl):S59–S69. doi: 10.1097/01.ta.0000219013.64168.b2. [DOI] [PubMed] [Google Scholar]
- 19.Beekley AC. Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med. 2008;36(7 suppl):S267–S274. doi: 10.1097/CCM.0b013e31817da7dc. [DOI] [PubMed] [Google Scholar]
- 20.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46:685–686. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 21.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 22.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 23.Spinella PC, Perkins JG, McLaughlin DF, et al. The effect of recombinant activated factor VII on survival in combat-related casualties with severe trauma requiring massive transfusion. J Trauma. 2008;64:286–293. doi: 10.1097/TA.0b013e318162759f. discussion 293–294. [DOI] [PubMed] [Google Scholar]
- 24.Stinger HK, Spinella PC, Perkins JG, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64(2 suppl):S79–S85. doi: 10.1097/TA.0b013e318160a57b. discussion S85. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 26.Grosso SM, Keenan JO. Whole blood transfusion for exsanguinating coagulopathy in a US field surgical hospital in postwar Kosovo. J Trauma. 2000;49:145–148. doi: 10.1097/00005373-200007000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Spinella PC, Perkins JG, Grathwohl KW. Risks associated with warm whole blood transfusions compared to PRBC transfusions during combat. Crit Care Med. 2005;331(12 Abstract supplement):A44. [Google Scholar]
- 28.Spinella PC, Moore FA, Holcomb JB, et al. Fresh whole blood tranfusions in coalition military, foreign national, and enemy combatant patients during Operation Iraqi Freedom at a US combat support hospital. World J Surg. 2008;32:255–261. doi: 10.1007/s00268-007-9201-5. [DOI] [PubMed] [Google Scholar]
- 29.Spinella PC. Warm fresh whole blood: military and civilian applications. Crit Care Med. 2008;36:S340–S345. doi: 10.1097/CCM.0b013e31817e2ef9. [DOI] [PubMed] [Google Scholar]
- 30.AAAM . Abbreviated Injury Scale. 1998 Version ed. Association for the Advancement of Automotive Medicine; Chicago, IL: 1998. [Google Scholar]
- 31.Holcomb JB, Wade CE, Michalek JE. Increased plasma and platelet to RBC ratios improves outcome in 466 massively transfused civilian trauma patients. 2008 doi: 10.1097/SLA.0b013e318185a9ad. Available at: http://www.americansurgical.info/abstracts/2008/16.cgi. [DOI] [PubMed]
- 32.Spinella PC, Perkins JG, Grathwohl KW, et al. Effect of plasma and red cell transfusions on survival for patients with combat related traumatic injuries. J Trauma. 2008;64(2 suppl):S69–S67. doi: 10.1097/TA.0b013e318160ba2f. discussion S77–S78. [DOI] [PubMed] [Google Scholar]
- 33.Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1182. doi: 10.1097/TA.0b013e31816c5c80. discussion 1182–1183. [DOI] [PubMed] [Google Scholar]
- 34.Maegele M, Lefering R, Paffrath T, Tjardes T, Simanski C, Bouillon B, Working Group on Polytrauma of the German Society of Trauma Surgery (DGU) Red blood cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiply injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie. Vox Sang. 2008;95:112–119. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 35.Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med. 2003;31(12 suppl):S687–S697. doi: 10.1097/01.CCM.0000099349.17094.A3. [DOI] [PubMed] [Google Scholar]
- 36.Napolitano LM, Corwin HL. Efficacy of red blood cell transfusion in the critically ill. Crit Care Clin. 2004;20:255–268. doi: 10.1016/j.ccc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Spinella PC, Perkins JG, Grathwohl KW, et al. Risks associated with fresh whole blood and red blood cell transfusions in a combat support hospital. Crit Care Med. 2007;35:2576–2581. doi: 10.1097/01.CCM.0000285996.65226.A9. [DOI] [PubMed] [Google Scholar]
- 38.Tinmouth A, Fergusson D, Yee IC, Hébert PC, ABLE Investigators. Canadian Critical Care Trials Group Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 39.Manno CS, Hedberg KW, Kim HC, et al. Comparison of the hemostatic effects of fresh whole blood, stored whole blood, and components after open heart surgery in children. Blood. 1991;77:930–936. [PubMed] [Google Scholar]
- 40.Mou SS, Giroir BP, Molitor-Kirsch EA, et al. Fresh whole blood versus reconstituted blood for pump priming in heart surgery in infants. N Engl J Med. 2004;351:1635–1644. doi: 10.1056/NEJMoa041065. [DOI] [PubMed] [Google Scholar]
- 41.Barbee RW, Kline JA, Watts JA. A comparison of resuscitation with packed red blood cells and whole blood following hemorrhagic shock in canines. Shock. 1999;12:449–453. doi: 10.1097/00024382-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald RD, Martin CM, Dietz GE, Doig GS, Potter RF, Sibbald WJ. Transfusing red blood cells stored in citrate phosphate dextrose adenine-1 for 28 days fails to improve tissue oxygenation in rats. Crit Care Med. 1997;25:726–732. doi: 10.1097/00003246-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Raat NJ, Verhoeven AJ, Mik EG, et al. The effect of storage time of human red cells on intestinal microcirculatory oxygenation in a rat isovolemic exchange model. Crit Care Med. 2005;33:39–45. doi: 10.1097/01.ccm.0000150655.75519.02. discussion 238–239. [DOI] [PubMed] [Google Scholar]
- 45.Sondeen J, Prince MD, Dubick MA, Holcomb JB. Fresh whole blood is the best 24 hour hypotensive resuscitation fluid in severe hemorrhagic shock. Shock. 2006;25:S121. [Google Scholar]
- 46.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17:223–231. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 47.Lavee J, Martinowitz U, Mohr R, et al. The effect of transfusion of fresh whole blood versus platelet concentrates after cardiac operations. A scanning electron microscope study of platelet aggregation on extracellular matrix. J Thorac Cardiovasc Surg. 1989;97:204–212. [PubMed] [Google Scholar]
- 48.Mohr R, Martinowitz U, Lavee J, Amroch D, Ramot B, Goor DA. The hemostatic effect of transfusing fresh whole blood versus platelet concentrates after cardiac operations. J Thorac Cardiovasc Surg. 1988;96:530–534. [PubMed] [Google Scholar]
- 49.Corwin HL, Gettinger A, Pearl RG, et al. The CRIT Study: anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit Care Med. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 50.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 51.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 52.Weinberg JA, McGwin G, Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–282. doi: 10.1097/TA.0b013e31817c9687. discussion 282–284. [DOI] [PubMed] [Google Scholar]
- 53.Utter GH, Reed WF, Lee TH, Busch MP. Transfusion-associated microchimerism. Vox Sang. 2007;93:188–195. doi: 10.1111/j.1423-0410.2007.00954.x. [DOI] [PubMed] [Google Scholar]
- 54.Nathens AB, Nester TA, Rubenfeld GD, Nirula R, Gernsheimer TB. The effects of leukoreduced blood transfusion on infection risk following injury: a randomized controlled trial. Shock. 2006;26:342–347. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]
- 55.Watkins TR, Rubenfeld GD, Martin TR, et al. Effects of leukoreduced blood on acute lung injury after trauma: a randomized controlled trial. Crit Care Med. 2008;36:1493–1499. doi: 10.1097/CCM.0b013e318170a9ce. [DOI] [PubMed] [Google Scholar]
- 56.Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–510. doi: 10.1097/00005373-199604000-00001. discussion 510–512. [DOI] [PubMed] [Google Scholar]
- 57.Hughes JD, Macdonald VW, Hess JR. Warm storage of whole blood for 72 hours. Transfusion. 2007;47:2050–2056. doi: 10.1111/j.1537-2995.2007.01429.x. [DOI] [PubMed] [Google Scholar]


