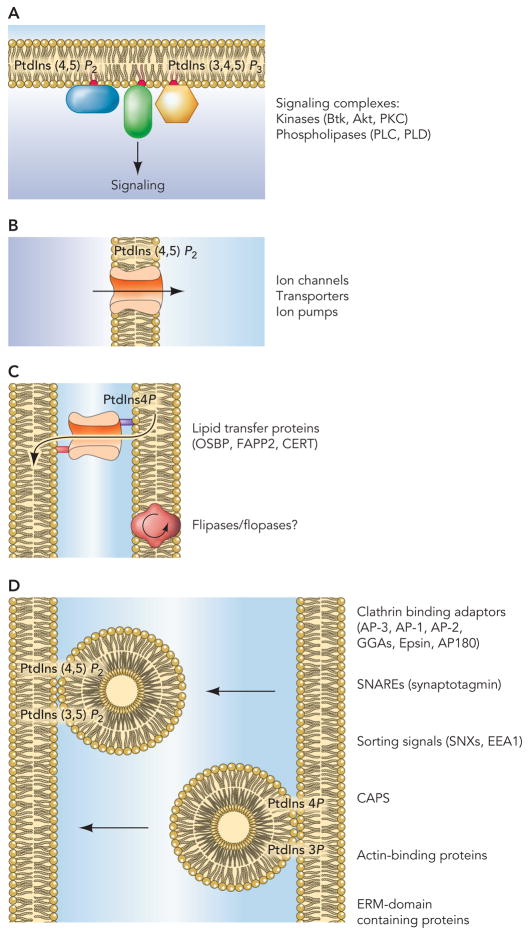
Figure 1.
Phosphoinositides regulate multiple membrane-associated molecular events. (A) Signaling from the inner leaflet of the plasma membrane is the best documented and known function of PtdIns(4,5)P2 and PtdIns(3,4,5)P3. PtdIns(4,5)P2 serves both as a precursor for PLC-generated messengers such as Ins(1,4,5)P3 and diacylglycerol, and an activator of other phospholiases, such as PLD. PtdIns(4,5)P2 is also converted to PtdIns(3,4,5)P3 by PI 3-kinases. This lipid recruits and activates several important protein kinases such as Akt/PKB, Btk and some PKC isoforms. (B) Phosphoinositides also regulate the activity of a number of ion channels and transporters thereby controlling ion distribution and gradients. (C) Phosphoinositides not only help hydrophilic and charged molecules (such as ions) to cross the hydrophobic membranes, but as increasingly being recognized, they also help hydrophobic molecules to traverse the aqueous phase separating the membranes. The lipid transfer proteins may carry their cargo to farther distances within the cell, but more likely they work within functional contact zones formed by adjacent membranes without moving too far from these membranes. Such functional zones exist between the ER and the Golgi, the ER and the mitochondria and the ER and the PM. Phosphoinositides probably also regulate the flipase and flopase proteins that help move certain lipids between the inner and the outer leaflet of membranes. (D) Phosphoinositides control the budding and fission process between membranes and hence are critical regulators of vesicular trafficking. Intriguingly the steady state phosphorylation state of phosphoinositides increases as one moves from the nuclear envelope/ER (very little if any phsophorylated PtdIns) toward the PM which has most of the PtdIns(4,5)P2 and PtdIns(3,4,5)P3. The role of phosphoinositides in these locations is to recruit adaptor proteins and to work together with the small GTP binding proteins often regulating their nucleotide exchange factors or GAP proteins.