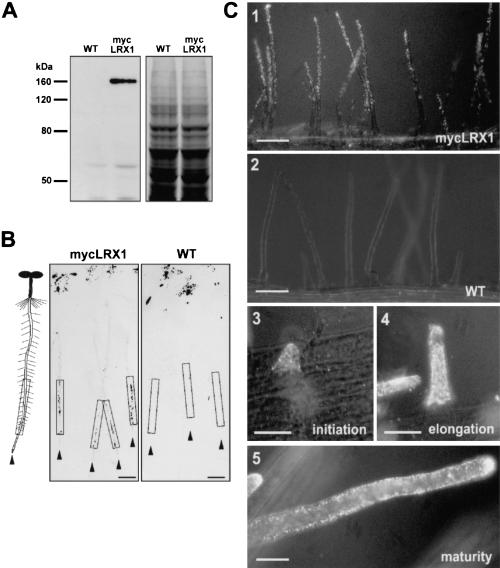
Figure 3.
Identification and immunolocalization of the LRX1 protein by epitope tagging. (A) Protein gel blot of root extracts from wild-type (WT) and transgenic (mycLRX1) plants expressing the c-myc-tagged LRX1 protein under control of the LRX1 promoter. Blots were incubated with the mouse myc-mAbs (left) and a duplicate gel was stained with Coomassie blue to test for equal loading (right). (B) Tissue print immunoblot. Four-day-old wild-type (WT) and transgenic mycLRX1 (mycLRX1) seedlings were pressed on nitrocellulose membranes to transfer soluble proteins. A scheme of seedlings at the same scale as the blots is represented at left of the panels for orientation. Arrowheads indicate the position of each root tip and frames delimit the root differentiation zone. The dark spots at the top of the membrane were caused by anthocyanins of the cotyledons also transferred onto the membrane. Signals were visible in the differentiation zone of mycLRX1 plants (mycLRX1) but not in controls (WT). Bars, 1 mm. (C) Whole-mount immunolocalization of mycLRX1. Three-day-old wild-type (WT) and mycLRX1 (mycLRX1) seedlings were fixed and immunolabeled with myc-mAbs. The c-myc epitope was found to be associated with root hairs in mycLRX1 (1), but not in wild-type (2) seedlings. Labeling was visible from root hair initiation (3), throughout root hair elongation (4), and after root hair maturation (5). Bars, 100 μm (1,2), 25 μm (3–5).