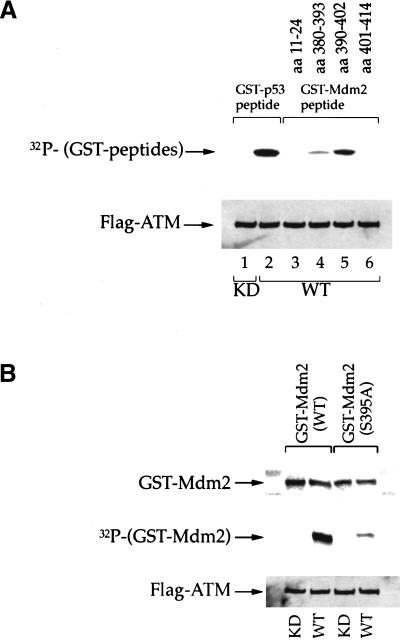
Figure 3.
Serine 395 is the major site on Mdm2 for phosphorylation by ATM in vitro. (A) Immunoprecipitated wild-type (WT) or kinase dead (KD) FLAG-ATM were incubated with a fixed amount of E. coli-expressed recombinant proteins, consisting of fusions between GST and peptides derived from various regions of human Mdm2. The amino acid positions corresponding to each peptide are indicated in the upper part of the panel (GST–Mdm2 peptide). Kinase assays were performed in vitro as described in Materials and Methods. A fusion between GST and a peptide corresponding to residues 9–21 of human p53 (GST–p53 peptide) was used as a positive control. Upper panel: 32P incorporation into the various GST peptides. Lower panel: Western blot with anti-FLAG M2 monoclonal antibody, confirming the presence of similar amounts of ATM protein in all reactions. (B) Immunoprecipitated WT or KD FLAG-ATM were incubated with recombinant proteins consisting of fusions between GST and full-length Mdm2, either wild-type or S395A. Kinase assays were performed in vitro as in A. Upper panel: Western blot with an Mdm2-specific monoclonal antibody, to assess the input amount of fusion protein in each reaction. Middle panel: 32P incorporation into the GST–Mdm2 fusion proteins. Lower panel: Western blot with anti FLAG M2 monoclonal antibody.