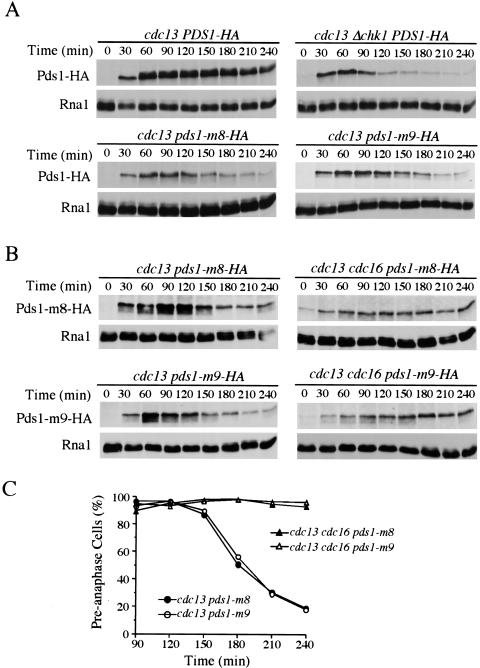
Figure 5.
Pds1-m8 and Pds1-m9 proteins are degraded by the APC in the presence of DNA damage. (A) Pds1-m8 and Pds1-m9 proteins are degraded in the presence of DNA damage. Y809 (cdc13-1), Y811 (cdc13-1 Δchk1), Y1074 (cdc13-1 pds1-m8), and Y1075 (cdc13-1 pds1-m9) cells expressing HA-tagged wild-type Pds1, Pds1-m8, or Pds1-m9 were synchronized in G1 with α-factor at 24°C. During the last 30 min of the α-factor block, cells were shifted to 32°C to inactivate cdc13-1. Cells were released into YPD at 32°C and α-factor was added to these cultures 55 min after the release to prevent cell cycle reentry. Protein samples were prepared at the indicated times, and Pds1 protein was analyzed by Western blotting. Levels of Rna1 are used as loading control. (B,C) Degradation of Pds1-m8 and Pds1-m9 mutant proteins is dependent on the APC, and apc mutants rescued the anaphase entry defect of pds1-m8 and pds1-m9 mutants in the presence of DNA damage. Y1074 (cdc13-1 pds1-m8), Y1083 (cdc13-1 cdc16-1 pds1-m8), Y1075 (cdc13-1 pds1-m9), and Y1084 (cdc13-1 cdc16-1 pds1-m9) cells were treated as in A except that cultures were shifted to 37°C instead of 32°C. Protein samples were prepared at the indicated times, and Pds1 protein was analyzed by Western blotting. Levels of Rna1 are used as a loading control. Aliquots also were withdrawn to examine DNA content by fluorescence-activated cell sorting and spindle morphology. Kinetics of anaphase entry is evaluated by the disappearance of short spindles (C).