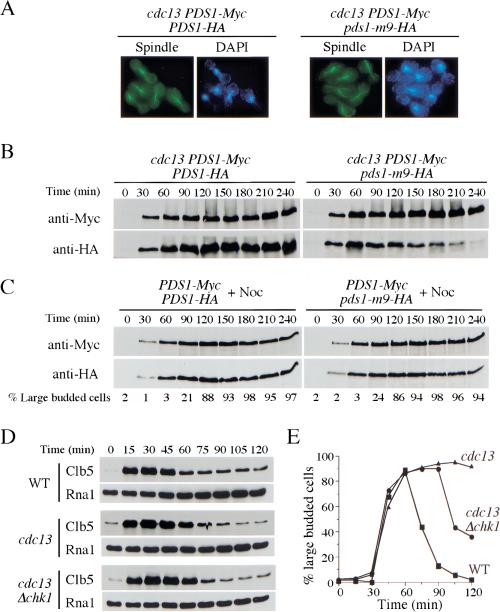
Figure 6.
APCCdc20 is active in the presence of DNA damage. (A,B) Pds1-m9 mutant protein is degraded during the preanaphase arrest maintained by wild-type Pds1 in the presence of DNA damage. Y1085 (cdc13-1 PDS1-Myc PDS1-HA) and Y1086 (cdc13-1 PDS1-Myc pds1-m9-HA) cells were synchronized in G1 with α-factor at 24°C. During the last 30 min of the α-factor block, cells were shifted to 32°C to inactivate cdc13-1. Then cells were released into YPD at 32°C. Aliquots were withdrawn at the indicated times to examine spindle morphology. Spindle morphology at 240 min after release indicated that cells maintained a preanaphase arrest (A). Protein samples also were prepared at the indicated times, and Pds1 protein was analyzed by Western blotting with both anti-Myc and anti-HA antibodies (B). (C) Pds1-m9 mutant protein is stabilized like wild-type Pds1 in the presence of spindle damage. Y1090 (PDS1-Myc PDS1-HA) and Y1091 (PDS1-Myc pds1-m9-HA) cells were synchronized in G1 with α-factor, then released into YPD with nocodazole (15 μg/mL) at 24°C. Protein samples were prepared at the indicated times, and Pds1 protein was analyzed by Western blotting with both anti-Myc and anti-HA antibodies. Aliquots also were withdrawn to examine the budding index. The percentage of large budded cells is shown below the Western blots. (D,E) Clb5 is degraded in the presence of DNA damage. Y1087 (wild type), Y1088 (cdc13-1), and Y1089 (cdc13-1 Δchk1) cells expressing HA-tagged Clb5 were synchronized in G1 with α-factor at 24°C. During the last 30 min of the α-factor block, cells were shifted to 34°C to inactivate cdc13-1. Cells were released into YPD at 34°C, and α-factor was added to these cultures 55 min after release to prevent cell cycle reentry. At the indicated times, protein samples were prepared to analyze the level of Clb5 protein, and aliquots were withdrawn to check the budding index. Levels of Rna1 are used as a loading control.