Abstract
Background
Chronic pain is a significant problem for many individuals following spinal cord injury (SCI). Unfortunately, SCI-related neuropathic pain has proven to be largely refractory to analgesic medications and other available treatments. Cranial electrotherapy stimulation (CES) has been effective in managing some types of pain. It involves the application of a small amount of current through the head via ear clip electrodes.
Objective
Explore the effectiveness of CES for neuropathic pain in persons with SCI and chronic pain.
Study design
Multi-site, double-blind, sham-controlled study.
Participants
Adults with SCI and chronic neuropathic pain at or below the level of injury were randomized to receive active or sham CES.
Intervention
Application of active CES or sham CES 1 hour daily for 21 days. Six-month open-label phase to assess ‘as-needed’ CES use.
Outcome measures
Change in pre- to post-session pain ratings as well as change in pain intensity, pain interference, pain quality, pain beliefs and coping strategies, general physical and mental health status, depressive symptomatology, perceived stress, and anxiety pre- to post-treatment.
Results
The active group reported a significantly greater average decrease in pain during daily treatments than the sham group (Kruskal–Wallis chi-square = 4.70, P < 0.05). During the 21-day trial, there was a significant group × time interaction for only one outcome variable; the active group showed larger pre- to post-treatment decreases in pain interference than the sham group did (F = 8.50, P < 0.01, d = 0.59).
Conclusions
On average, CES appears to have provided a small but statistically significant improvement in pain intensity and pain interference with few troublesome side effects. Individual results varied from no pain relief to a great deal of relief.
Keywords: Neuropathic, Musculoskeletal, Spinal cord injuries, Analgesia, Pregabalin, Gabapentin, Acupuncture, Cranial electrotherapy stimulation, Opioids
Introduction
Chronic pain is a significant problem for many individuals following spinal cord injury (SCI) and may have a major impact on their function and quality of life.1 For example, Nepomuceno et al.2 reported that 80% of 200 persons with SCI reported abnormal sensations and 48% reported sensations that were painful. Turner et al.3 similarly found that 79% of 384 persons with SCI experienced pain. Sixty-one percent reported bilateral pain below the level of injury, 41% reported pain above the injury, and 50% reported pain at the level of injury. Rintala et al.4 reported that 75% of 77 men with SCI reported chronic pain and more than 25% of these had more than one chronic pain component. These investigators also noted that the presence of chronic pain was associated with more depressive symptoms, more perceived stress, and poorer self-assessed health. In reviewing eight recent studies, Siddall and Loeser5 calculated that, when averaging across the studies, 65% of persons with SCI report having pain.
Many persons with SCI and chronic pain report that their pain is severe. For example, 25% of the sample in the study by Nepomuceno et al.2 reported their pain to be severe or extreme and 44% stated that it had increased over time. In the Siddall and Loeser review5 mentioned above, across all eight studies, an average of nearly one-third rated their pain as severe. The average pain intensity ratings provided by participants in a study by Widerstrom-Noga et al.6 was 5.6 on a 0–10 scale.
Pain in persons with SCI has been treated with a large number of biomedical and psychosocial interventions. However, the efficacy of these treatments has remained largely inconclusive and SCI-related pain has proven to be largely refractory to treatment. For example, Nepomuceno et al.2 reported that 38% of those with pain used medications to relieve the pain, but only 22% reported that they obtained consistent pain relief through the use of analgesics. Turner et al.3 found that less than half of their sample had ever used opioids for pain, even though opioids were the only treatment included on the checklist other than implanted morphine pump with an average perceived effectiveness rating greater than 3 out of a maximum of 5. Gabapentin was not included on the checklist, but was added by 14 respondents (4.6% of the 304 participants with pain; mean helpfulness = 3.21). Alternative treatments that were not included in the checklist, but were written in by the survey respondents, included massage (n = 16, mean helpfulness = 3.63), acupuncture (n = 11, mean helpfulness = 3.09), and marijuana (n = 8, mean helpfulness = 4.38). The prevalence of use of these added treatments cannot be ascertained from these findings since it is possible that not everyone who had used them wrote them in.
More recent reviews of the evidence for pharmacological intervention for SCI neuropathic pain7,8 reveal a significant number of publications over the past 10 years suggesting efficacy for a variety of agents. The strongest evidence appears to exist for the use of pregabalin in a study by Siddall et al.9 On average, the group who received pregabalin reported a nearly two-point decrease in pain ratings on a 0–10 numeric rating scale, whereas the placebo group reported an average decrease of less than half a point. Furthermore, about 42% of patients who received pregabalin reported substantial (>30%) relief compared to only 16% for those on placebo.
Cranial electrotherapy stimulation (CES): CES is a non-traditional therapy involving the application of a small amount of current, usually less than 1 mA, through the head via ear clip electrodes. The analgesic action of subperception CES has been demonstrated in various anti-nociception models.10–12 Extracellular recording techniques indicated that CES modifies noxious evoked responses in pain-processing regions of the brains of rats.13,14 In humans, the mechanism of action of CES is not fully understood; however, it has been shown to ‘normalize’ neurotransmitter homeostasis,15 stimulate the hypothalamic–pituitary axis by increasing IGF-1 production (R. B. Smith and C. A. Ryser, ‘The use of CES in anti-aging medicine: Great things we learn when research goes wrong,’ presented at the International Oxidative Medicine Association meeting, Westminster, CO, 18 March 2000), bring neurotransmitters in stressed participants back to normal levels of homeostasis (M. S. Gold, A. L. Pottash, Sternbach, Barbaban, and Asunitto, ‘Anti-withdrawal effect of alpha methyl dopa and cranial electrotherapy,’ presented at the Society for Neuroscience meeting, Minneapolis, MN, 31 October 1982), and increase beta-endorphins in patients with chronic back pain.16
CES has been found to be effective in controlling anxiety, depression, insomnia, and generalized stress.17 A study by Capel et al.18 found that the intensity of pain of mixed types decreased significantly more in 15 participants with SCI who received active CES treatment twice a day for 2 hours for each of 4 consecutive days compared to 15 participants who received sham CES for the same amount of time. To further explore the use of CES in persons with SCI, we conducted a double-blind, sham-controlled, pilot study in 2002–2003 in which 38 veterans with SCI with neuropathic or musculoskeletal pain were randomly assigned to either CES (n = 18) or sham CES (n = 20).19 They were trained to self-administer the CES treatment at home and to complete pain ratings on a 0–10 scale immediately before and after each treatment session. The treatment group received 1 hour per day of 100 µA sub-sensation CES for a total of three consecutive weeks (21 days). The control group received sham CES for the same amount of time. After completion of the treatment, participants in the control group were offered an open-label use of the active device for 21 days. They again completed pain ratings before and after each daily session. The average change in pain on a scale from 0 to 10 from before to after each session across the 21 days was −0.73 (SD = 1.15) points for the active CES treatment group and −0.08 (SD = 0.38) points for the sham treatment group. This was a significant difference (tseparate = 2.27, P < 0.05, Cohen's d effect size = 0.76). Furthermore, 17 of the 20 participants from the group that originally had the sham treatment chose to participate in the subsequent open-label phase, and they reported a small but statistically significant pre- to post-session decrease in pain intensity (mean before = 5.97, mean after = 5.51, t = 3.47, P < 0.01). The participants in both groups also completed the Brief Pain Inventory (BPI) adapted for persons with disabilities20 before and after the 21-day treatment phase. The group who received the active CES treatment reported a significant decrease in pain interference (pre- and post-treatment BPI interference scores = 58.89 and 44.33 (SDs = 26.93 and 31.18, respectively); t = 3.31, P < 0.01). However, there was no significant change in pain interference for the group who received sham CES (pre- and post-BPI interferences scores = 53.10 and 48.40, respectively, (SDs = 27.88 and 28.03, respectively); t = 1.22, P > 0.20), a reduction of 4.7 points.
Building upon these pilot findings, a multisite study, funded by the Veterans Affairs Rehabilitation Research and Development Service, involving four Veterans Affairs medical facilities and one private rehabilitation center was conducted from 2004 to 2008. This paper reports the outcomes of that study. Approval of the study was obtained from the Institutional Review Board for Baylor College of Medicine and Affiliated Hospitals, the Office of the Institutional Review Board for Human Use at the University of Alabama at Birmingham, the Edward Hines, Jr. Veterans Affairs Hospital and North Chicago Veterans Affairs Medical Center Institutional Review Board, and the Louis Stokes Cleveland Veterans Affairs Medical Center Institutional Review Board.
The primary hypothesis of the study was that active CES will significantly reduce pain intensity more than sham CES from before to after the daily treatment sessions. The secondary hypotheses were that from before to after the 21-day trial: (1) active CES will significantly reduce pain intensity and pain interference as well as significantly impact pain quality, pain beliefs, and pain coping strategies more than sham CES and (2) active CES will significantly reduce health-related disability and psychological distress (depressive symptomatology, stress, and anxiety) more than sham CES.
The study was also designed to answer the following questions in secondary analyses: (1) what side effects, if any, will the participants in the active and sham CES conditions experience? and (2) what proportion of the participants will elect to participate in a long-term (6-month) open-label phase following initial short-term (21 days) use of active CES? For participants in the long-term component, we also wanted to determine: (1) how long and how frequently will they use the device during the 6-month phase and what problems will they encounter with the device? and (2) how satisfied will the participants be with CES and would they plan to continue to use CES if they could keep the device longer?
Method
Design
This was a multi-site, double-blind, sham-controlled study. Candidates who met the inclusion and exclusion criteria were randomly assigned to receive active CES treatment or sham CES treatment. The sample size was determined by using Statistica95 software to ensure adequate power for testing the primary hypothesis based on the findings from our pilot study. Specifically, 64 subjects for the active CES treatment group and 64 subjects for the sham CES treatment group were required to test our primary hypothesis regarding average change in pain ratings from before to after the 21 daily sessions. This sample size was based on the following assumptions: a mean change of 0.05 in the sham group and 0.37 in the active CES group, a SD of 0.65, an effect size of 0.50, an alpha of 0.05, and power of 0.8. Thus, a total of 128 participants who complete the study were required. Allowing for 25% attrition, 172 participants were to be recruited.
Outcome measures
Measures consisted of a demographic data sheet, as well as measures assessing pain intensity and pain interference (Pain Intensity and Pain Interference Subscales of the BPI adapted for persons with disability20 and the Pain subscale of the Medical Outcomes Study 36-item Short Form Health Survey (SF-36)21), pain quality (Paroxysmal, Deep, and Surface Pain subscales of the Pain Quality Assessment Scale (PQAS)22), pain beliefs and pain coping (Maladaptive and Adaptive Coping subscales of the Two-Item Measures of Pain Beliefs and Coping Strategies23,24), health-related disability (Physical and Mental Component Summaries (PCS & MCS, respectively) of the Medical Outcomes Study 12-item Short Form Health Survey (SF-12)25), and distress (10-item short form of the Center for Epidemiologic Studies-Depression scale (CES-D-10),26 10-item Perceived Stress Scale (PSS-10),27 and the Short-Form State-Trait Anxiety Inventory (SF-STAI-6)28).
Equipment
The CES equipment used in this study was the Alpha-Stim SCS (Electromedical Products International Inc., Mineral Wells, TX, USA), which is a prescription medical technology that is FDA approved for the management of pain, anxiety, depression, and insomnia.
Participants
Inclusion criteria: Participants had to be at least 18 years old, have a SCI (any level and any degree of completeness) that had occurred at least 6 months prior to study entry. They had to report at least one chronic (at least 6-months duration) pain component at or below the level of injury which was classified as primarily neuropathic pain and was rated as at least a 5 on a pain intensity scale ranging from 0 to 10.
Exclusion criteria: Potential participants were excluded if they had an active substance abuse problem that would interfere with participation, a serious psychological or psychiatric disorder, or an implanted electrical device.
Recruitment: Persons with SCI who met the inclusion and exclusion criteria were recruited from four treatment sites with access to a large number of persons with SCI – Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas; Spain Rehabilitation Center at the University of Alabama at Birmingham in collaboration with the Birmingham Veterans Affairs Medical Center; Edward J. Hines, Jr. Veterans Affairs Hospital in Hines, Illinois; and Louis Stokes Veterans Affairs Medical Center in Cleveland, Ohio. The investigators at each site recruited potential candidates in person when a candidate was seen as a patient, through Institutional Review Board (IRB)-approved flyers placed at the various sites, or by telephone for persons who gave their consent in an earlier study to be contacted for future research projects. Prior to contacting potential candidates by telephone, a letter was sent that briefly explained the project. The consent form and copies of the study questionnaires (see below) were included to allow the person to make an informed decision about participating. The research coordinator explained the study to the potential candidates. Those interested in participating, who met the study criteria, signed an IRB-approved consent form. An investigator at each site determined whether the candidate had neuropathic pain at or below the level of injury based on responses to the Modified (Self-report) Leeds Neuropathic Symptoms and Signs Scale (S-LANSS)29 and other clinical questions.
Procedure
Once the potential study participant was found to meet all inclusion criteria, he or she was randomly assigned to either a control group (sham CES) or a treatment group (active CES). The equipment was set up for a double-blind study by the manufacturer such that the participants could not differentiate active from sham CES devices. Research staff members who interacted with the participants (e.g. recruited and trained participants, administered questionnaires, followed up by telephone) did not know which devices were sham and which were active. Randomization was achieved by selecting a device from a box initially containing equal numbers of active and sham devices.
The research coordinators provided training on the use of the device and administered the pre-intervention packet of questionnaires at their respective medical centers. Alternatively, for participants who could not easily come to the medical center, they administered the packet of questionnaires by telephone and mailed the CES device, a training video (DVD or videotape as desired by the participant), instruction sheets, and forms to the participant and contacted the participant by telephone to be sure the procedure was clearly understood. They instructed the participant to use the device at home for the next 21 days and to use the Daily Pain Rating Sheet to monitor the intensity of their at- or below-level neuropathic pain immediately before and after each treatment session. Persons in the treatment group received 1 hour per day of 100 µA sub-sensation active CES. Those in the control group received sham CES for the same amount of time. The research coordinator telephoned each participant weekly during the three-week period to assure that the participant was complying with the protocol and to identify any problems and side effects.
After the 21 days, the participant returned the device to the medical center and completed a packet of post-intervention questionnaires. If the participant was unable to come to the medical center on the 22nd day, the research coordinator completed the post-intervention questionnaire by telephone. The research coordinator then checked with the person who had the code indicating which devices were active and which were shams to determine to which group the participant had been assigned. Those in the sham group were given the opportunity to try an active device (open-label) for three more weeks. Participants who decided to use the open-label device were instructed to again complete the daily pain ratings immediately before and after each session. Unlike the blinded phase, in which the current was pre-set to a sub-threshold level of 100 µA, during the open-label phase of the study, participants were able to select the current setting ranging from 1 to 5 (100–500 µA). The selected current setting for each session was recorded on the daily pain rating sheet. After the 21-day open-label phase, the participants returned the device and completed a second set of post-intervention questionnaires.
Participants, who completed the 21-day active treatment, either initially or in the open-label phase for the sham group, were given the opportunity to participate in a 6-month open-label phase of the study. Persons who elected to do so were instructed to use the device whenever they wished. They were not required to use the device everyday. However, they were asked to complete a Pain Rating Sheet before and after each session, indicating the date of each session. The research coordinator contacted them by telephone monthly to identify and correct problems. At the end of 3 and 6 months, they completed a set of questionnaires similar to those previously administered as well as an additional questionnaire designed to determine their perceptions of the device. Participants received $25 each time for completing questionnaire packets at intake, post-initial treatment, and post-3-week-open-label treatment (if applicable) and $10 each time for completing questionnaire packets at 3 and 6 months during the long-term phase.
Data analysis
Descriptive statistics were obtained for characteristics of the sample and outcome measures including mean, SD, range and skewness for continuous variables, and frequency and percentage for categorical variables. Chi-square analyses and t-tests were used to compare the active and sham groups on these measures.
The primary hypothesis (active CES will significantly reduce pain intensity more than sham CES from before to after the daily treatment sessions) was tested using a non-parametric Kruskal–Wallis analysis of variance (ANOVA). A non-parametric test was required because the average change in pain from before to after daily treatment sessions was substantially skewed (skewness = −1.87). The dependent variable was average change in pain from before to after daily treatment sessions and the independent variable was treatment group (active or sham). Paired t-tests were also performed to determine change within each treatment group from before to after treatment.
The secondary hypotheses regarding the effect of active CES, relative to sham CES, on pain intensity, pain interference, pain quality, pain beliefs, and pain coping strategies were tested using a series of repeated measures ANOVA, with the dependent variables being the Pain Intensity and Interference subscales of the BPI, the Pain subscale of the SF-36, the Paroxysmal, Deep, and Surface subscales of the PQAS, and the Maladaptive and Adaptive Coping subscales of the Two-Item Measures of Pain Beliefs and Coping Strategies. The independent variables were treatment group, time (before or after treatment), and a product representing the group × time interaction effect. Paired t-tests were performed to determine change within groups if there was a significant group × time interaction.
The secondary hypotheses regarding the effect of active CES, relative to sham CES, on health-related disability and psychological distress were tested using the same methods that were used to test the secondary hypotheses concerning pain, except that in these analyses, the dependent variables were health-related quality of life (SF-12 PCS and MCS), depressive symptomatology (CES-D-10), perceived stress (PSS-10), and state anxiety (SF-STAI-6). The results of all secondary analyses were considered preliminary because of the large number of analyses and associated risk for Type I error.
Bivariate correlations and chi-square analyses were used to identify predictors of participation in the 6-month phase. Potential predictors included age, race/ethnicity, initial assignment to active or sham treatment groups, baseline and pre-long-term phase pain intensity, change in pain during the 21-day active CES trial and mental and physical health measures. Change across time (baseline, after active CES, after 3 and 6 months in the long-term phase) in average pain intensity as measured by the BPI was assessed with repeated-measures ANOVAs. Other analyses of the long-term 3- and 6-month data were descriptive (proportion of eligible individuals electing to participate in the 6-month phase, number of uses of CES per month, change or stability of pain ratings before and after each session across time, problems encountered including side effects, and participant satisfaction with the device).
Results
Descriptive analyses
One hundred eleven eligible persons completed consent forms and were enrolled into the study. Enrollment stopped short of the planned 172 subjects because funding for the study ended before all of the planned subjects could be recruited. Of the subjects enrolled into the study, the record of group assignment was lost for 6 persons; thus those 6 persons were not included in any analyses. Characteristics of the remaining 105 participants (46 active, 59 sham) are displayed in Table 1. There were no significant differences between the active and sham groups on any of the demographic or SCI characteristics.
Table 1.
Characteristics of the sample
| Characteristic | Active | Sham | P |
|---|---|---|---|
| Number | 46 | 59 | |
| Age (years: mean ± SD (range)) | 52.1 ± 10.5 (27–79) | 52.5 ± 11.7 (26–80) | 0.854 |
| Male gender (n (%)) | 38 (82.6) | 52 (88.1) | 0.575 |
| Race/ethnicity (n (%)) | 0.577 | ||
| White, not Hispanic | 37 (80.4) | 44 (74.6) | |
| African–American | 9 (19.6) | 14 (23.7) | |
| Hispanic | 0 (0.0) | 1 (1.7) | |
| High-school education or less (n (%)) | 13 (28.3) | 22 (37.3) | 0.406 |
| Living with spouse or significant other (n (%)) | 22 (47.8) | 38 (64.4) | 0.113 |
| Time since onset of SCI (years: mean, SD, (range)) | 14.6 ± 9.5 (1–33) | 15.8 ± 12.1 (1–46) | 0.579 |
| Time from onset of SCI to onset of neuropathic pain (n (%)) | 0.591 | ||
| < 1 month | 18 (39.1) | 22 (37.3) | |
| 1–12 months | 14 (30.4) | 24 (40.7) | |
| 1–5 years | 8 (17.4) | 9 (15.3) | |
| 6 years or more | 6 (13.0) | 4 (6.8) | |
| Level and completeness of SCI (n (%)) | 0.678 | ||
| Tetraplegia complete | 5 (10.9) | 5 (8.5) | |
| Tetraplegia incomplete | 14 (30.4) | 12 (20.3) | |
| Tetraplegia, completeness unknown | 0 (0.0) | 1 (1.7) | |
| Paraplegia complete | 14 (30.4) | 18 (30.5) | |
| Paraplegia incomplete | 10 (21.7) | 16 (27.1) | |
| Paraplegia, completeness unknown | 3 (6.5) | 5 (8.5) | |
| Level and completeness unknown | 0 (0.0) | 2 (3.4) |
SD, standard deviation; SCI, spinal cord injury.
Effect of CES on pain intensity during daily treatment sessions (primary hypothesis)
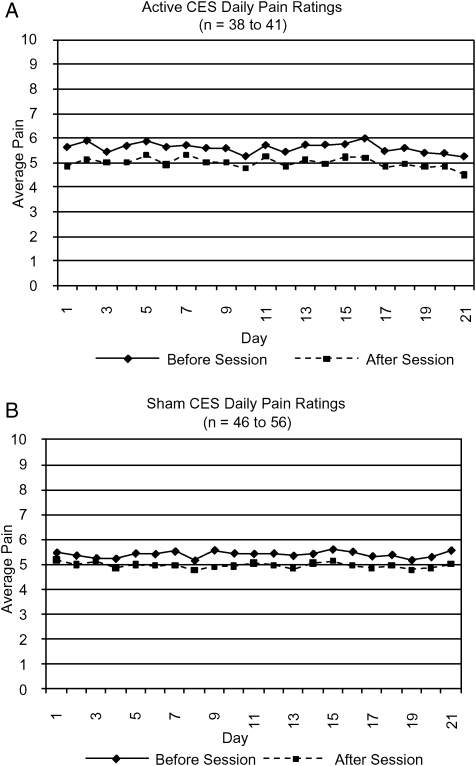
Blinded phase: Forty-one of the 46 persons assigned to the active group and 56 of the 59 persons assigned to the sham group completed at least some daily pain ratings (6–21 days, mean = 20.1) during the 3-week blinded phase. The active and sham groups did not differ significantly on average daily pain ratings before (means: active = 5.60 (SD = 1.78), sham = 5.41 (SD = 1.85), P > 0.60) or after (means: active = 5.00 (SD = 1.92), sham = 5.00 (SD = 1.93), P > 0.90) treatment. Daily pain ratings before and after each session for the active and sham groups are displayed in Fig. 1. The amount of change in pain did not differ much from day to day within either group and there was no significant linear trend for either the before or after ratings across the 21 days for either group. However, based on the non-parametric Kruskal–Wallis test for the primary hypothesis, the active group had a significantly greater average decrease in pain from before to after the daily treatments compared to the sham group (mean rank: active 41.82, sham 54.26; chi-square = 4.70, P < 0.05). A lower rank indicates a greater decrease in pain because a decrease was indicated by a negative number. The paired t-test within the active group yielded a mean pain intensity decrease of 0.60 points on the 0–10 scale (SD = 0.83, t = 4.66, P < 0.001), yielding a Cohen's d effect size of 0.73, which is in the high–moderate range.30 For the sham group, the mean decrease was 0.40 points (SD = 1.03, t = 2.94, P < 0.01), yielding a low–moderate effect size (d = 0.49).
Figure 1.
Average daily pain ratings before and after CES sessions for (A) active and (B) sham groups.
Open-label phase: Forty persons initially assigned to the sham group provided at least some daily pain ratings (1–21 days, mean = 19.4) for the 3-week open-label phase. The mean current setting selected was 419 (SD = 90, range 100–500). A paired t-test of the average before-session (mean = 5.34, SD = 1.84) and after-session (mean = 4.55, SD = 1.92) ratings yielded a significant reduction in pain (mean difference = 0.80, t = 4.11, P < 0.001, d = 0.42). Of these 40 persons, 23% reported an average decrease in pain intensity of at least 30% of the before-session rating compared with only 10% during the blinded phase.
Effect of CES on pain outcome measures during the 21-day trial
Blinded phase: Forty-five of the 46 persons assigned to the active group and 55 of the 59 persons assigned to the sham group completed the post-blinded phase questionnaires. Descriptive summaries of the data from the outcome measures for these 100 participants are displayed in Table 2. The active and sham groups differed significantly at baseline on three of the pain outcome measures despite randomization – BPI Pain Interference (t = 3.60, P < 0.001), SF-36 Pain (t = 2.54, P < 0.05), and Maladaptive Coping (t = 3.17, P < 0.001). In each case, the active group had ‘poorer’ baseline scores (lower for SF-36 Pain and higher for BPI Pain Interference and Maladaptive Coping).
Table 2.
Pre- and post-treatment measures for blinded and open-label phases
| Blinded phase |
Open-label phase |
|||||
|---|---|---|---|---|---|---|
| Active group n = 45 |
Sham group n = 55 |
Sham group n = 40 |
||||
| Pre | Post | Pre | Post | Pre | Post | |
| Pre–post pain measures (mean ± SD) | ||||||
| BPI pain intensity subscale | 23.9 ± 5.5 | 21.6 ± 6.6 | 23.4 ± 6.0 | 20.8 ± 6.7 | 21.8 ± 6.0** | 20.8 ± 5.9** |
| BPI pain interference subscale* | 56.2 ± 21.7*** | 39.5 ± 24.3*** | 38.5 ± 27.3*** | 32.2 ± 23.8*† | 31.6 ± 23.9 | 28.1 ± 23.7 |
| 36-item short-form health survey (SF-36) pain subscale* | 36.0 ± 17.8 | 41.4 ± 16.6 | 44.9 ± 17.1 | 52.5 ± 18.6 | 51.3 ± 20.2 | 49.3 ± 18.9 |
| PQAS paroxysmal pain subscale | 5.6 ± 2.0 | 4.5 ± 2.1 | 4.9 ± 1.9 | 4.3 ± 2.2 | 4.2 ± 2.1 | 4.1 ± 2.0 |
| PQAS surface pain subscale | 4.1 ± 2.5 | 3.6 ± 2.1 | 3.4 ± 2.0 | 3.4 ± 2.0 | 3.5 ± 1.9 | 3.4 ± 1.7 |
| PQAS deep pain subscale | 4.5 ± 2.5 | 3.8 ± 2.3 | 3.8 ± 2.3 | 3.4 ± 2.3 | 3.3 ± 2.3 | 3.5 ± 2.2 |
| Two-item measures of pain beliefs and coping strategies maladaptive coping subscale* | 37.4 ± 11.7 | 30.6 ± 13.5 | 29.5 ± 13.1 | 26.6 ± 14.3 | 26.6 ± 14.4 | 25.6 ± 13.2 |
| Two-item measures of pain beliefs and coping strategies adaptive coping subscale | 30.2 ± 11.3 | 28.9 ± 10.2 | 29.2 ± 12.5 | 30.0 ± 12.0 | 30.8 ± 9.7 | 32.6 ± 9.0 |
| Pre–post health measures (mean ± SD) | ||||||
| 12-item short-form health survey (SF-12) physical component summary (PCS) | 28.7 ± 20.5 | 27.8 ± 21.6 | 34.5 ± 23.4 | 33.6 ± 20.7 | 35.7 ± 23.5 | 37.5 ± 25.9 |
| 12-item short-form health survey (SF-12) mental component summary (MCS)* | 55.3 ± 26.5 | 59.1 ± 24.1 | 67.5 ± 22.5 | 66.2 ± 23.7 | 64.3 ± 24.7 | 63.7 ± 25.5 |
| 10-item center for epidemiologic studies depression scale (CES-D-10)* | 13.4 ± 6.9 | 11.9 ± 6.9 | 10.2 ± 6.0 | 9.9 ± 6.0 | 9.8 ± 6.1 | 9.3 ± 5.8 |
| 10-item perceived stress scale (PSS-10) | 16.4 ± 7.4 | 13.9 ± 6.9 | 14.0 ± 7.4 | 13.0 ± 7.6 | 12.9 ± 7.5 | 12.3 ± 7.6 |
| 6-item short-form state-trait anxiety inventory (SF-STAI-6)* | 11.2 ± 4.2 | 11.1 ± 4.0 | 9.2 ± 3.8 | 9.4 ± 3.6 | 9.2 ± 4.0 | 9.8 ± 4.3 |
*Measures on which active and sham groups were significantly different at pre-treatment in the blinded phase.
Within-group paired t-tests: **P ≤ 0.05, ***P ≤ 0.001, *†P ≤ 0.01.
Note: Lower scores are better in all measures except those from the SF-36 and SF-12.
In repeated measures ANOVAs, among the eight pre–post pain measures, there were significant main effects of time for six of the eight measures, including BPI Intensity (F = 29.66, P < 0.001, d = 0.40), BPI Interference (F = 42.16, P < 0.001, d = 0.44), SF-36 Pain (F = 15.16, P < 0.001, d = 0.37), PQAS Paroxysmal Pain (F = 19.88, P < 0.001, d = 0.41), PQAS Deep Pain (F = 9.50, P < 0.01, d = 0.22), and Maladaptive Coping (F = 18.12, P < 0.001, d = 0.35). There were no significant effects for time on the PQAS Surface Pain and Adaptive Coping scales.
Only BPI Interference (F = 7.37, P < 0.01, d = 0.55), SF-36 Pain (F = 10.35, P < 0.01, d = 0.65), and Maladaptive Coping (F = 6.23, P < 0.05, d = 0.51) showed a significant main effect of group and only BPI Interference showed a significant group × time interaction effect (F = 8.50, P < 0.01, d = 0.59), with the active treatment group showing larger pre- to post-treatment decreases in pain interference than the sham group. Because the group × time interaction was significant for BPI Pain Interference, paired t-tests within each group were performed for this measure. Both the active (t = 6.02, P < 0.001, d = 0.73) and sham (t = 2.80, P < 0.01, d = 0.25) groups showed significant improvement in pain interference, but there was less improvement in the sham group.
Open-label phase: Of the 55 participants who were assigned to the sham group and who provided post-treatment questionnaire data in the blinded phase, 40 provided questionnaire data following a 3-week open-label phase (Table 2). Paired t-tests were performed to assess change in the pain outcome measures from pre- to post-treatment during the open-label phase. The only significant change among the eight pain measures was a significant reduction in BPI Pain Intensity (t = 2.08, P < 0.05, d = 0.17).
Effect of CES on health outcome measures during the 21-day trial
Blinded phase: Again in spite of randomization, the two groups were initially different on three of the five measures of health (Table 2), including the SF-12 MCS (t = 2.44, P < 0.05), the CES-D-10 (t = 2.45, P < 0.05), and the SF-STAI-6 (t = 2.54, P < 0.05). In each case, the active group had ‘poorer’ scores (lower for SF-12 MCS and higher for CES-D-10 and SF-STAI-6). In repeated-measures ANOVAs, among the five pre–post measures of health, only the PSS-10 had a significant main effect of time (F = 8.60, P < 0.01, d = 0.22). Only the SF-12 MCS (F = 2.44 m P < 0.05, d = 0.44), the CES-D-10 (F = 2.45, P < 0.05, d = 0.44), and the SF-STAI-6 (F = 2.54, P < 0.05, d = 0.52) had a main effect of group. These overall group differences may be due to the initial group differences on these measures. There were no significant time × group interactions for any of the five health variables.
Open-label phase: There was no significant change in any of the five health measures during the open-label treatment.
Side effects
Comments regarding side effects were recorded by participants at each session. The number of people reporting various side effects during the blinded phase is shown in Table 3. We report on the number of people who endorsed the side effects rather than number of times a side effect was reported because it is unclear whether all participants recorded an effect each time it occurred. As can be seen, there were no serious study-related adverse events in any phase of the study. Most common in both groups were various sensations such as tingling on the ears and drowsiness. The number of different side effects and the types of side effects reported were not significantly related to the number of sessions completed during the 21 days. Two participants in the sham group completed only six sessions, one of whom reported five different side effects and the other reported two different side effects. All 54 other participants in the sham group completed at least 16 sessions and all 41 of those in the active group completed at least 18 sessions regardless of number or type of side effects reported.
Table 3.
Side effects during blinded phase
| Side effect | Active | Sham |
|---|---|---|
| Number | 41 | 56 |
| Ears pulse, tingle, sting, itch, small electric feeling, ear clips too tight | 12 | 6 |
| Head tingles | 0 | 1 |
| Electric shot in feet, burning in legs, legs tingling | 1 | 1 |
| Spasms, leg spasms | 1 | 2 |
| Burning in buttocks | 1 | 0 |
| Ringing in ears | 1 | 0 |
| Drowsy, sleepy, fell asleep, relaxing | 7 | 4 |
| Dizzy, lightheaded, feeling crooked | 3 | 1 |
| Nausea, stomach rolled | 1 | 2 |
| Shaky | 0 | 1 |
| Heart racing, chest pain | 0 | 2 |
| Headache, slight headache | 2 | 3 |
| Metallic or unusual taste in mouth | 1 | 1 |
| Increased pain | 2 | 1 |
Note: Counts reflect number of people reporting effect at least once.
Long-term open-label phase
Effect of CES on pain intensity during treatment sessions: Eighty-six (82%) of the 105 participants were eligible for participation in the 6-month long-term open-label phase because they had completed a period of active CES, either initially in the blinded phase or in the 3-week open-label phase. Of those eligible, 39 persons (45%) provided at least some pre–post session data during the 6-month phase. Fifty percent of those eligible assigned to the active group provided session data during the long-term phase, compared to 27% of eligible persons initially assigned to the sham group. The number of days during the 6-month phase in which the device was used ranged from 5 to 186 (mean = 88.6, SD = 58.5) and the average current setting was 396 µA (SD = 14.58) out of a possible 500 µA. The number of participants reporting use in any given month ranged from 18 in month 6 to 38 in month 1. Within each of the 6 months, for persons reporting any use in a given month, the average number of days used was approximately 20 (range 1–31 days) or roughly two-thirds of the days in that month on average.
For participants who used the device at least once during the 6 months, the total number of days the device was used was significantly inversely correlated to depressive symptomatology (CES-D-10, n = 38, r = −0.41, P < 0.05) and perceived stress (PSS-10, n = 38, r = −0.41, P < 0.05) as measured just prior to the long-term phase. Thus, participants with less depressive symptomatology and less perceived stress were likely to use the device more often than those with more depressive symptomatology and stress. Frequency of use was not significantly related to demographic characteristics or measures of pain, changes in pain, or health obtained either at study entry or at the beginning of the 6-month phase.
Change in pain during sessions: Thirty participants provided at least some session data in each of the first three months of the long-term phase. For these 30 persons, the average before- to after-session decrease in pain was relatively stable (1.37, 1.46, and 1.45 points, respectively, on a 0–10 scale). However, for the 13 persons who provided at least some session data for all 6 months of the long-term phase, the average session decrease declined over time (1.12, 1.14, 0.94, 0.79, 0.73, and 0.57 points on the 0–10 scale for the decreases in pain during the first through the sixth month of the long-term phase, respectively).
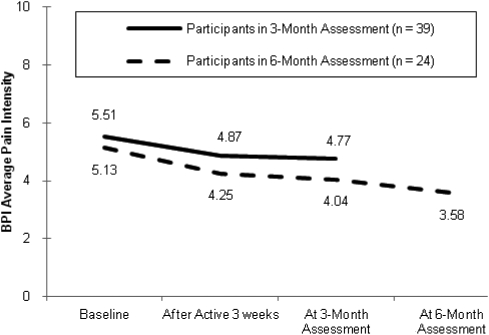
End of month 3 in the long-term open-label phase: Thirty-nine participants provided ratings on the BPI Average Pain Intensity item at the end of the third month of the long-term phase. For these 39 persons, average pain intensity decreased from baseline to the end of active CES, either initially or during the 3-week open-label phase, and this decrease was maintained during the first 3 months of the long-term phase (Fig. 2). The main effect of time across the three time points was significant (F = 5.69, P < 0.01, d = 0.48). There was also a significant linear trend (F = 7.35, P < 0.01).
Figure 2.
BPI average pain intensity across time (3-month assessment in the long-term phase: main effect of time – P < 0.01, linear trend – P < 0.01; 6-month assessment in the long-term phase: main effect of time – P < 0.001, linear trend – P < 0.001).
End of month 6 in the long-term open-label phase: For the 24 persons who also provided BPI data at the end of the sixth month of the long-term phase, the decrease in average pain intensity continued to be maintained (Fig. 2). The main effect of time across the four data points was significant (F = 10.50, P < 0.001, d = 1.31) and there was also a significant linear trend (F = 27.59, P < 0.001).
Participant perceptions of CES: At 3 (n = 41) and 6 (n = 26) months during the 6-month long-term phase participants completed questionnaires regarding their perceptions of CES. At 3 months 56% and at 6 months 85% of the respondents who provided satisfaction ratings were somewhat or very satisfied with the device; assuming that the participants who did not provide ratings were not satisfied with the CES device, the overall rates of satisfaction (of all 105 participants) are 22 and 21%, respectively. At 3 months, among those who provided ratings and who were still using the device, 51% reported that the device relieved pain at least a moderate amount, 41% reported that it improved mental health, and 71% said they would continue to use the device if they could keep it (these percentages are 19, 15, and 26%, respectively, for the participants as a whole, assuming that those not providing ratings and/or not using the CES device at these times would not find it helpful for reducing pain or improving mental health). Similarly, at 6 months, 54% of the respondents reported at least moderate pain relief, 46% reported at least a moderate improvement in mental health, and 68% said they would continue to use the device if they could keep it (13, 11, and 16% of all 105 participants, respectively). When asked what they liked best about the device, the most common response was that it was easy to use. When asked what they liked least about it, the most common responses were that it did not help the pain and it made the ears tingle or hurt.
Discussion
Chronic pain following SCI is a common problem that deeply affects the quality of life. CES is one of a number of non-pharmacological treatments that has a significant effect on pain in persons with SCI. In a pilot study of 38 veterans with neuropathic or musculoskeletal pain, participants receiving active CES had a significantly greater decrease in daily pain following CES exposure as compared to those persons receiving sham exposure.19 Even though the decrease in pain in the active group of that study was relatively small, the effect size was medium to large (Cohen's d = 0.73).
As in the pilot study, in the present multi-site study, the group receiving active CES had a relatively small, but statistically significant, decrease in pain, on average, after daily sessions of CES. Although in the current study, the sham group also had a significant decrease in pain after the CES sessions, the decrease in pain for the active group was significantly greater than that for the sham group when comparing change using the non-parametric Kruskal–Wallace test. An open-label trial for those originally in the sham group also found a small, but statistically significant, decrease in pain after daily sessions of CES further supporting the findings of the blinded trial. These significant effects were found despite the fact that the current study was underpowered to detect such effects, given the power analysis performed prior to the study's initiation.
The findings suggest that although CES treatment may have a small (but reliable) effect on pain intensity from before to after a session, it does not appear to have a longer-lasting effect on pain intensity from one day to the next. On the other hand, although pain interference with daily life decreased significantly over the 21-day trial in both groups, the active group reported a greater decrease in pain interference, suggesting the possibility that CES may have a beneficial impact on this domain. This is an important finding because reduction in pain interference (or improvement in daily functioning) is often regarded by many clinicians as being a more meaningful outcome than reduction in pain intensity.
Although the findings indicate that CES has a statistically significant effect on pain intensity, statistical significance is not equivalent to clinical significance. In the area of pain research, the commonly used criterion for clinical significance is a 30% or more reduction in pain.31 In this study very few (<14%) participants in either group achieved this degree of pain reduction.
Recent reviews of the evidence for pharmacological intervention for SCI neuropathic pain7,8 reveal a significant number of publications over the past 10 years suggesting efficacy for a variety of agents. The strongest evidence exists for the use of pregabalin, with roughly a 2-point average decrease in pain ratings on a scale of 0–10 compared to a half-point decrease for placebo.9 Given that neither pregabalin, nor any of the other agents currently available can offer a complete elimination of pain, having a cadre of different methodologies is desirable, including CES, which could potentially complement each other and produce more manageable levels of pain.
While attrition was very high in this study by the end of the third and sixth months of the long-term phase (>55% and >70%, respectively) there was a decrease in pain intensity as a function of time reported by those participants still enrolled. The high attrition rate may account for the discrepancy between the significant reduction of average pain intensity across time for participants in the 3- and 6-month long-term assessments (Fig. 2), and the lack of significant pre–post change in pain intensity for the total number of participants in the initial 21-day blinded or open-label phases. Those participants who did not experience pain reductions may have been more likely to terminate their participation prior to the assessments at the end of the third and sixth months of the long-term phase. Furthermore, the amount of electric current received was self-selected. On average, they received 396 µA out of a possible 500 µA during the long-term phase compared with a sub-sensation 100 µA during the blinded phase. Overall, compliance was strong for those remaining in the study with regard to the use of the device, with participants in any given month using the device approximately 2 out of every 3 days, on average, for the duration of the 6-month trial.
Consistent with the pilot study, there were no unexpected adverse events caused by CES treatment. The most common side effects in both the active and sham groups were a pulsing, tingling, stinging, itching, and/or a small electric feeling produced by the ear clips. The findings also indicated that among those eligible to participate in the long-term phase, roughly twice as many of those who had received active treatment (as compared to those who had received the sham treatment initially) chose to do so. Furthermore, those who completed the 3- and 6-month long-term assessments continued to show significantly more improvement in pain reduction across the 6-month period. Although the reason for the continued improvement remains unclear, the findings suggest the possibility that among a subgroup of individuals, the CES treatment could potentially have a long-term therapeutic impact. This possibility should be examined in future research.
Some important limitations of this study should be noted. One limitation is the baseline differences between active and sham groups on several of the outcome measures, thus, making group differences in change scores difficult to interpret. In future studies, a stratification scheme for randomization to ensure similarity between treatment conditions at baseline would help to alleviate this problem. Another limitation of this study is the amount of attrition by the end of the 6-month phase. Less than 38% of the original study participants completed the 3-month assessments and less than 23% completed the 6-month assessments. This made it impossible to evaluate the long-term efficacy of the treatment for the individuals who withdrew. However, the results do indicate that some individuals will continue to use the device on an as-needed basis for at least 6 months, and that some improvement can be seen in pain intensity over time for those who do use it. It could be expected that those individuals who obtain some relief are more likely to continue a treatment if there are no unacceptable side effects. An additional limitation of the current study is that all of the outcome measures were obtained by self-report. The addition of some objective measures of outcome, such as a rating of function made by the person's health-care provider or a family member, would strengthen the findings.
Overall, the participants reported that they were satisfied with the device, specifically the ease of use, and many reported that they would be likely to continue to use the device if they had access to it. The primary complaint by some participants was that it was not actually reducing pain as much as they would have liked. However, for a subset of the participants, CES appeared to be at least somewhat effective, and there were minimal side effects. Future studies could include a 6-month trial in which participants are required to use CES everyday for 1 hour at a standardized current.
Conclusion
On average, CES appears to have provided a small but statistically significant improvement in pain intensity and pain interference with few troublesome side effects in a sample of individuals with SCI and chronic pain. However, there was variability in treatment response; some persons experienced no pain relief while others reported a great deal of relief. It is important to learn what personal and/or pain characteristics are predictive of the effectiveness of CES. Until that information is available, and since side effects are minimal, the findings indicate that a trial of CES for persons with SCI who have not yet found effective treatment for their neuropathic pain at or below the level of injury may be warranted.
Acknowledgement
We thank all the research assistants and coordinators for their time and effort in implementation of this research study. We also thank Daniel L. Kirsch, PhD for his assistance in the use of the CES devices. The study was funded by the Veterans Affairs Rehabilitation Research and Development Service. Electromedical Products International, Inc., Mineral Wells, Texas, provided the active and sham CES devices and the necessary batteries, ear clip pads, and wetting solution.
None of the authors have a conflict of interest with the manufacturer of the CES devices.
References
- 1.Tan G, Young S. Pain-related psychosocial and vocational issues in rehabilitation. In: Monga TN, Grabois M. (eds.) Pain management in rehabilitation. New York: Demos Medical Publishing; 2002. pp. 35–57 [Google Scholar]
- 2.Nepomuceno C, Fine PR, Richards JS, Gowens H, Stover SL, Rantanabol U, et al. Pain in patients with spinal cord injury. Arch Phys Med Rehabil 1979;60(12):605–9 [PubMed] [Google Scholar]
- 3.Turner JA, Cardenas DD, Warms CA, McClellan CB. Chronic pain associated with spinal cord injuries: a community survey. Arch Phys Med Rehabil 2001;82(4):501–9 [DOI] [PubMed] [Google Scholar]
- 4.Rintala DH, Loubser PG, Castro J, Hart KA, Fuhrer MJ. Chronic pain in a community-based sample of men with spinal cord injury: prevalence, severity, and relationship with impairment, disability, handicap, and subjective well-being. Arch Phys Med Rehabil 1998;79(6):604–14 [DOI] [PubMed] [Google Scholar]
- 5.Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord 2001;39(2):63–73 [DOI] [PubMed] [Google Scholar]
- 6.Widerstrom-Noga EG, Felipe-Cuervo E, Yezierski RP. Relationships among clinical characteristics of chronic pain after spinal cord injury. Arch Phys Med Rehabil 2001;82(9):1191–7 [DOI] [PubMed] [Google Scholar]
- 7.Wrigley P, Siddall PJ. Pharmacological interventions for neuropathic pain following spinal cord injury: an update. Top Spinal Cord Inj Rehabil 2007;13(2):58–71 [Google Scholar]
- 8.Teasell RW, Mehta S, Aubut JA, Foulon B, Wolfe DL, Hsieh JT, et al. A systematic review of pharmacologic treatments of pain after spinal cord injury. Arch Phys Med Rehabil 2010;91(5):816–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology 2006;67(10):1792–800 [DOI] [PubMed] [Google Scholar]
- 10.Wilson OB, Hamilton RF, Warner RL, Johnston CM, deFriece R, Harter L, et al. The influence of electrical variables on analgesia produced by low current transcranial electrostimulation of rats. Anesth Analg 1989;68(5):673–81 [PubMed] [Google Scholar]
- 11.Capel ID, Dorrell HM, Spencer EP. The application of sub-perception electrical stimuli elicits a temporally distinct response from restraint stress: I. antinociceptive characteristics. J Bioelectricity 1990;9(2):167–76 [Google Scholar]
- 12.Gabis L, Shklar B, Baruch YK, Raz R, Gabis E, Geva D. Pain reduction using transcranial electrostimulation: a double blind “active placebo” controlled trial. J Rehabil Med 2009;41(4):256–61 [DOI] [PubMed] [Google Scholar]
- 13.Qiao JT, Skolnick M, Dafny N. Dorsal raphe and external electrical stimulation modulate noxious input to single neurons in nucleus parafascicularis thalami. Brain Res Bull 1988;21(4):671–75 [DOI] [PubMed] [Google Scholar]
- 14.Dong WQ, Wilson OB, Skolnick MH, Dafny N. Hypothalamic, dorsal raphe and external electrical stimulation modulate noxious evoked responses of habenula neurons. Neuroscience 1992;48(4):933–40 [DOI] [PubMed] [Google Scholar]
- 15.Pozos RS, Strack LF, White RK, Richardson AW. Electrosleep versus electroconvulsive therapy. In: Reynolds DV, Sjoberg AE. (eds.) Neuroelectric research. Springfield, IL: Charles Thomas; 1971. pp. 221–5 [Google Scholar]
- 16.Gabis L, Shklar B, Geva D. Immediate influence of transcranial electrostimulation on pain and beta-endorphin blood levels: an active placebo-controlled study. Am J Phys Med Rehabil 2003;82(2):81–5 [DOI] [PubMed] [Google Scholar]
- 17.Kirsch DL, Smith RB. The use of cranial electrotherapy stimulation in the management of chronic pain: a review. Neurorehabilitation 2000;14(2):85–94 [PubMed] [Google Scholar]
- 18.Capel ID, Dorrell HM, Spencer EP, Davis MW. The amelioration of the suffering associated with spinal cord injury with subperception transcranial electrical stimulation. Spinal Cord 2003;41(2):109–17 [DOI] [PubMed] [Google Scholar]
- 19.Tan G, Rintala DH, Thornby JI, Yang J, Wade W, Vasilev C. Using cranial electrotherapy stimulation to treat pain associated with spinal cord injury. J Rehabil Res Dev 2006;43(4):461–74 [DOI] [PubMed] [Google Scholar]
- 20.Engel JM, Schwartz L, Jensen MP, Johnson DR. Pain in cerebral palsy: the relation of coping strategies to adjustment. Pain 2000;88(3):225–30 [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30(6):473–83 [PubMed] [Google Scholar]
- 22.Victor TW, Jensen MP, Gammaitoni AR, Gould EM, White RE, Galer BS. The dimensions of pain quality: factor analysis of the Pain Quality Assessment Scale. Clin J Pain 2008;24(6):550–5 [DOI] [PubMed] [Google Scholar]
- 23.Jensen MP, Keefe FJ, Lefebvre JC, Romano JM, Turner JA. One- and two-item measures of pain beliefs and coping strategies. Pain 2003;104(3):453–69 [DOI] [PubMed] [Google Scholar]
- 24.Tan G, Nguyen Q, Cardin SA, Jensen MP. Validating the use of two-item measures of pain beliefs and coping strategies for a veteran population. J Pain 2006;7(4):252–60 [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–33 [DOI] [PubMed] [Google Scholar]
- 26.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med 1994;10(2):77–84 [PubMed] [Google Scholar]
- 27.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24(4):385–96 [PubMed] [Google Scholar]
- 28.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992;31(Pt 3):301–6 [DOI] [PubMed] [Google Scholar]
- 29.Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain 2005;6(3):149–58 [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988 [Google Scholar]
- 31.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9(2):105–21 [DOI] [PubMed] [Google Scholar]