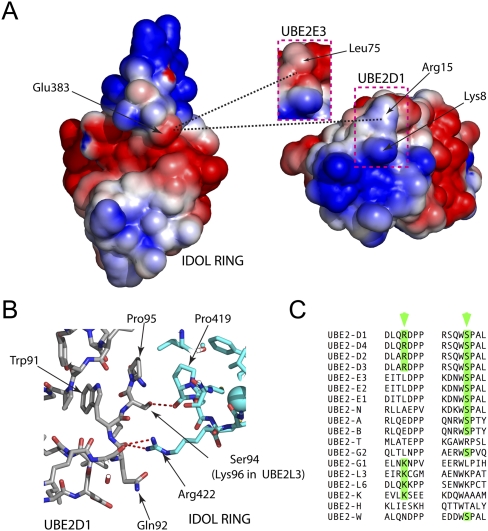
Figure 5.
Specificity determinants for the IDOL RING:UBE2D interaction. (A) Electrostatic potential of the interface between the IDOL RING domain (left) and UBE2D1 (right). Note that the main interaction surface on the E2 is highly basic, and the complementary surface on the E3 is acidic. Arg15 in UBE2D1 provides a basic pocket to accommodate Glu383 from IDOL. (Insert) In noncomplementary E2s such as UBE2E3, the residue in this position is neutral or acidic and disfavors interaction. (B) Some E2s that are noncomplementary with IDOL have a basic residue in position 15, but an important serine at the interface (Ser94 in UBE2D1) is substituted with other amino acids, such as lysine in UBE2L3. The serine makes an important backbone contact that could not be formed by the alternative residues. (C) Alignment of key regions of various E2 ligases. Only members of the UBE2D family have both a basic residue and a serine to support appropriate interactions with the IDOL RING.