Figure 5.
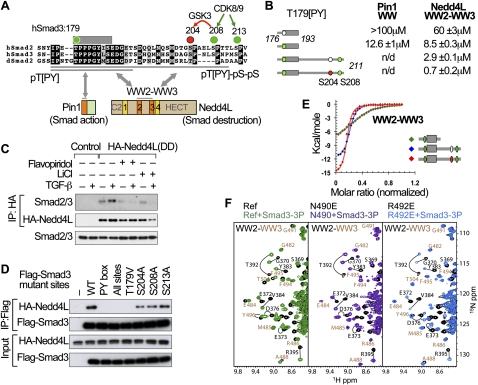
Elements of a Smad action turnover switch in the TGF-β pathway. (A) Sequence alignment of the linker regions of human Smad2 and Smad3 and Drosophila Smad2, with the conserved residues highlighted. The conserved CDK8/9 sites (green) and CDK8/9-primed GSK3 site (red) and the PY box are shown. Two Smad3 segments (176–193 and 176–211) used in this study are underlined. The domain composition of Pin1 and Nedd4L proteins and the regions that mediate binding to linker phosphorylated Smad3 are indicated. (B) Synthetic Smad3 (phospho-)peptides and their affinity for the recombinant Pin1 WW domain and Nedd4L WW2–WW3 pair. Colored circles denote phosphorylation of the indicated residues. (n.d.) Not determined. (C) TGF-β-dependent formation of a complex between HA-Nedd4L(DD) and endogenous Smad3, and effects of flavopiridol and LiCl on the formation of this complex. (D) Effect of alanine mutations in the PY box and the indicated phosphorylation sites on the ability of Flag-tagged Smad3 constructs to bind HA-Nedd4L(DD) in HEK293 cells. (E) ITC curves and corresponding fitting to pairs of Nedd4L WW domains and the indicated Smad3 (phospho-)peptides. (F) NMR titrations of WW2–WW3 pairs (wild type in green) with point mutations introduced in two residues that coordinate the pS204pS208 site (violet and royal blue). Residues that belong to WW2 and WW3 are labeled in black and camel, respectively.