Figure 7.
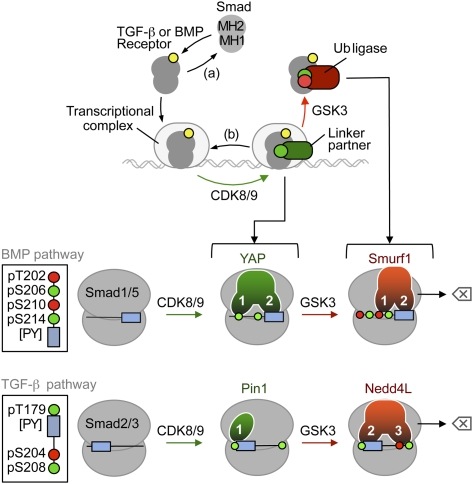
The Smad action turnover switch in the BMP and TGF-β pathways: pSer codes and WW domain code readers. (Top panel) Schematic summary of the Smad action turnover switch in the BMP and TGF-β pathways. Following receptor-mediated phosphorylation (yellow circle), Smad proteins translocate to the nucleus and assemble transcriptional complexes, which are phosphorylated at CDK8/9 sites (green circle) in the MH1–MH2 interdomain linker region. This phosphorylation creates high-affinity binding sites for transcriptional partners (such as YAP in the case of the BMP mediator Smad1, Pin1 in the case of the TGF-β mediator Smad3, and probably others), thus achieving peak transcriptional action. Phosphorylation by CDK8/9 also primes the Smads for GSK3-mediated phosphorylation (red symbol) at the −4 position, which favors the binding of ubiquitin ligases Smurf1 (BMP pathway) and Nedd4L (TGF-β pathway), leading to proteasome-dependent degradation of Smad molecules that participate in transcription (erase symbol). Alternatively, C-terminal Smad phosphatases (a) and linker phosphatases (b) reverse these phosphorylation states. See the text for details and citations. (Bottom panels) Schematic of the Smad linker phospho-amino acid codes (insets) and WW domain code readers. The conserved CDK8/9 phosphorylation sites (green circles) and GSK3 sites (red circles) are located at the indicated positions relative to the PY box (slate box). Amino acid positions correspond to Smad1 and Smad3. In the BMP pathway, the YAP WW1 domain binds to pS206 in Smad1, as long as p210 is not phosphorylated. The Smurf1 WW1 domain binds with higher affinity to the pS210–pS214 motif. The WW2 domains bind the [PY] motif. In the TGF-β pathway, the sole WW domain of Pin1 binds the pT179[PY] motif, as does the WW2 domain of Nedd4L. However, the Nedd4L WW3 domain increases the binding affinity by recognizing the pS204–pS208 motif. See the text for additional details and citations on the known roles of these WW domain proteins in Smad signal transduction.